- 1.
Yue Pan, Shiyu Zhen, Xiaozhi Liu, Mengshu Ge, Jianxiong Zhao, Lin Gu, Dan Zhou, Liang Zhang, Dong Su, "Looping metal-support interaction in heterogeneous catalysts during redox reactions", Nat Commun, 16, 8627, 2025.
@article{Pan2025b,
title = {Looping metal-support interaction in heterogeneous catalysts during redox reactions},
author = {Yue Pan and Shiyu Zhen and Xiaozhi Liu and Mengshu Ge and Jianxiong Zhao and Lin Gu and Dan Zhou and Liang Zhang and Dong Su},
doi = {10.1038/s41467-025-63646-1},
issn = {2041-1723},
year = {2025},
date = {2025-12-00},
urldate = {2025-12-00},
journal = {Nat Commun},
volume = {16},
number = {8627},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
 2.
2.Xiangfu Niu, Yanjun Chen, Mingze Sun, Satoshi Nagao, Yuki Aoki, Zhiqiang Niu, Liang Zhang, "Bayesian-Learning-Assisted Catalyst Discovery for Efficient Iridium Utilization in Electrochemical Water Splitting", Science Advances, 11, 34, 2025, eadw0894.
@article{nokey,
title = {Bayesian-Learning-Assisted Catalyst Discovery for Efficient Iridium Utilization in Electrochemical Water Splitting},
author = {Xiangfu Niu, Yanjun Chen, Mingze Sun, Satoshi Nagao, Yuki Aoki, Zhiqiang Niu, Liang Zhang},
url = {https://www.science.org/doi/10.1126/sciadv.adw0894},
year = {2025},
date = {2025-08-20},
urldate = {2025-08-20},
journal = {Science Advances},
volume = {11},
issue = {34},
pages = {eadw0894},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
 3.
3.Xiangfu Niu, Shiyu Zhen, Rui Zhang, Jianqiu Li, Liang Zhang, "Unraveling Disorder-to-Order Transitions and Chemical Ordering in PtCoM Ternary Alloys Using Machine Learning Potential", Chemical Science , 16, 33, 2025, 14884-14893.
@article{nokey,
title = {Unraveling Disorder-to-Order Transitions and Chemical Ordering in PtCoM Ternary Alloys Using Machine Learning Potential},
author = {Xiangfu Niu, Shiyu Zhen, Rui Zhang, Jianqiu Li, Liang Zhang},
url = {https://doi.org/10.1039/D5SC04043D},
year = {2025},
date = {2025-07-15},
urldate = {2025-07-15},
journal = {Chemical Science },
volume = {16},
issue = {33},
pages = {14884-14893},
note = {selected},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
4.Xiangfu Niu, Shuwei Li, Zheyu Zhang, Haohong Duan, Rui Zhang, Jianqiu Li, Liang Zhang, "Accelerated Optimization of Compositions and Chemical Ordering for Bimetallic Alloy Catalysts Using Bayesian Learning", ACS Catalysis, 2025, 4374-4383.
@article{Niu2025,
title = {Accelerated Optimization of Compositions and Chemical Ordering for Bimetallic Alloy Catalysts Using Bayesian Learning},
author = {Xiangfu Niu and Shuwei Li and Zheyu Zhang and Haohong Duan and Rui Zhang and Jianqiu Li and Liang Zhang},
url = {https://doi.org/10.1021/acscatal.5c00467},
doi = {10.1021/acscatal.5c00467},
year = {2025},
date = {2025-02-26},
urldate = {2025-02-26},
journal = {ACS Catalysis},
pages = {4374-4383},
publisher = {American Chemical Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
 5.
5.Zhe He, Kailang Li, Tianxiang Chen, Yunchao Feng, Eduardo Villalobos-Portillo, Carlo Marini, Tsz Woon Benedict Lo, Fuyuan Yang, Liang Zhang, Lichen Liu, "High-purity hydrogen production from dehydrogenation of methylcyclohexane catalyzed by zeolite-encapsulated subnanometer platinum-iron clusters", Nature Communications, 16, 1, 2025, 92.
@article{He2025,
title = {High-purity hydrogen production from dehydrogenation of methylcyclohexane catalyzed by zeolite-encapsulated subnanometer platinum-iron clusters},
author = {Zhe He and Kailang Li and Tianxiang Chen and Yunchao Feng and Eduardo Villalobos-Portillo and Carlo Marini and Tsz Woon Benedict Lo and Fuyuan Yang and Liang Zhang and Lichen Liu},
url = {https://doi.org/10.1038/s41467-024-55370-z},
doi = {10.1038/s41467-024-55370-z},
issn = {2041-1723},
year = {2025},
date = {2025-01-02},
urldate = {2025-01-02},
journal = {Nature Communications},
volume = {16},
number = {1},
pages = {92},
abstract = {Liquid organic hydrogen carriers (LOHCs) are considered promising carriers for large-scale H2 storage and transportation, among which the toluene-methylcyclohexane cycle has attracted great attention from industry and academia because of the low cost and its compatibility with the current infrastructure facility for the transportation of chemicals. The large-scale deployment of the H2 storage/transportation plants based on the toluene-methylcyclohexane cycle relies on the use of high-performance catalysts, especially for the H2 release process through the dehydrogenation of methylcyclohexane. In this work, we have developed a highly efficient catalyst for MCH dehydrogenation reaction by incorporating subnanometer PtFe clusters with precisely controlled composition and location within a rigid zeolite matrix. The resultant zeolite-encapsulated PtFe clusters exhibit the up-to-date highest reaction rate for dehydrogenation of methylcyclohexane to toluene, very high chemoselectivity to toluene (enabling the production of H2 with purity >99.9%), remarkably high stability (>2000þinspaceh) and regenerability over consecutive reaction-regeneration cycles.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Liquid organic hydrogen carriers (LOHCs) are considered promising carriers for large-scale H2 storage and transportation, among which the toluene-methylcyclohexane cycle has attracted great attention from industry and academia because of the low cost and its compatibility with the current infrastructure facility for the transportation of chemicals. The large-scale deployment of the H2 storage/transportation plants based on the toluene-methylcyclohexane cycle relies on the use of high-performance catalysts, especially for the H2 release process through the dehydrogenation of methylcyclohexane. In this work, we have developed a highly efficient catalyst for MCH dehydrogenation reaction by incorporating subnanometer PtFe clusters with precisely controlled composition and location within a rigid zeolite matrix. The resultant zeolite-encapsulated PtFe clusters exhibit the up-to-date highest reaction rate for dehydrogenation of methylcyclohexane to toluene, very high chemoselectivity to toluene (enabling the production of H2 with purity >99.9%), remarkably high stability (>2000þinspaceh) and regenerability over consecutive reaction-regeneration cycles.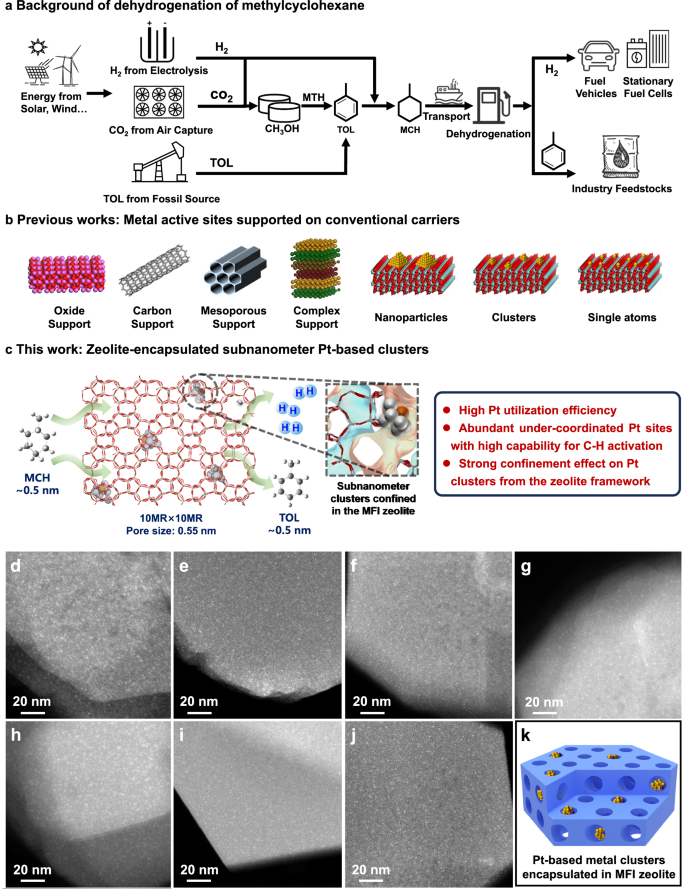 6.
6.Shuwei Li, Liang Zhang, "Accurate first-principles simulation for the response of 2D chemiresistive gas sensors", npj Comput Mater, 10, 1, 2024.
@article{Li2024,
title = {Accurate first-principles simulation for the response of 2D chemiresistive gas sensors},
author = {Shuwei Li and Liang Zhang},
doi = {10.1038/s41524-024-01329-z},
issn = {2057-3960},
year = {2024},
date = {2024-06-29},
urldate = {2024-06-29},
journal = {npj Comput Mater},
volume = {10},
number = {1},
publisher = {Springer Science and Business Media LLC},
abstract = {<jats:title>Abstract</jats:title><jats:p>The realm of chemiresistive gas sensors has witnessed a notable surge in interest in two-dimensional (2D) materials. The advancement of high-performance 2D gas sensing materials necessitates a quantitative theoretical method capable of accurately predicting their response. In this context, we present our first-principles framework for calculating the response of 2D materials, incorporating both carrier concentration and mobility. We showcase our method by applying it to prototype NH<jats:sub>3</jats:sub> sensing on 2D MoS<jats:sub>2</jats:sub> and comparing the results with prior experiments in the literature. Our approach offers a thorough solution for carrier concentration, taking into account the electronic structure around the Fermi level. In conjunction with the mobility calculation, this enables us to provide a quantitative prediction of the response profile and limit of detection (LOD), yielding a notably improved alignment with prior experimental findings. Further analysis quantifies the contributions of carrier concentration and mobility to the overall response of 2D MoS<jats:sub>2</jats:sub> to NH<jats:sub>3</jats:sub>. We identify that discrepancies in the charge-transfer-based method primarily stem from overestimating carrier concentrations. Our method opens exciting opportunities to explore carrier mobility-dominated sensing materials, facilitates efficient screening of promising gas sensing materials, and quantitative understanding of the sensing mechanism.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
<jats:title>Abstract</jats:title><jats:p>The realm of chemiresistive gas sensors has witnessed a notable surge in interest in two-dimensional (2D) materials. The advancement of high-performance 2D gas sensing materials necessitates a quantitative theoretical method capable of accurately predicting their response. In this context, we present our first-principles framework for calculating the response of 2D materials, incorporating both carrier concentration and mobility. We showcase our method by applying it to prototype NH<jats:sub>3</jats:sub> sensing on 2D MoS<jats:sub>2</jats:sub> and comparing the results with prior experiments in the literature. Our approach offers a thorough solution for carrier concentration, taking into account the electronic structure around the Fermi level. In conjunction with the mobility calculation, this enables us to provide a quantitative prediction of the response profile and limit of detection (LOD), yielding a notably improved alignment with prior experimental findings. Further analysis quantifies the contributions of carrier concentration and mobility to the overall response of 2D MoS<jats:sub>2</jats:sub> to NH<jats:sub>3</jats:sub>. We identify that discrepancies in the charge-transfer-based method primarily stem from overestimating carrier concentrations. Our method opens exciting opportunities to explore carrier mobility-dominated sensing materials, facilitates efficient screening of promising gas sensing materials, and quantitative understanding of the sensing mechanism.</jats:p>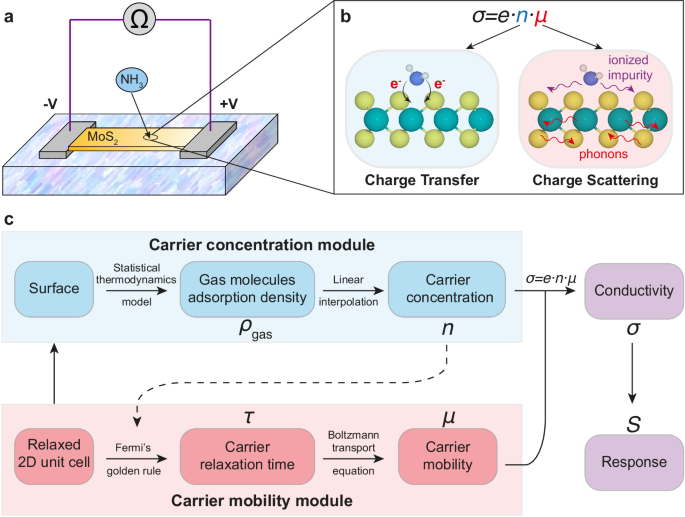 7.
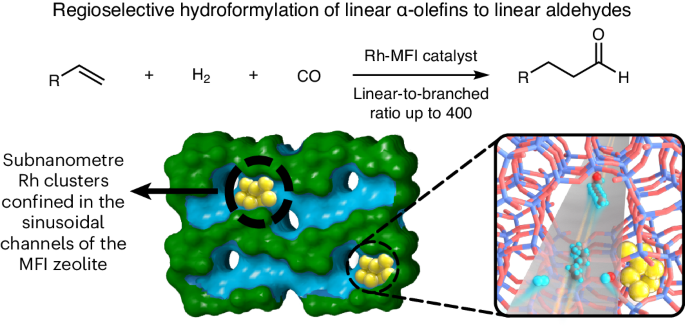
7.Xiaomeng Dou, Tao Yan, Lixiang Qian, Huaming Hou, Miguel Lopez-Haro, Carlo Marini, Giovanni Agostini, Debora M Meira, Xiangjie Zhang, Liang Zhang, Zhi Cao, Lichen Liu, "Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite", Nature Catalysis, 7, 6, 2024, 666-677.
@article{Dou1929b,
title = {Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite},
author = {Xiaomeng Dou and Tao Yan and Lixiang Qian and Huaming Hou and Miguel Lopez-Haro and Carlo Marini and Giovanni Agostini and Debora M Meira and Xiangjie Zhang and Liang Zhang and Zhi Cao and Lichen Liu},
url = {https://zhanglab-thu.com/wp-content/uploads/2024/05/s41929-024-01155-y-3.pdf},
doi = {10.1038/s41929-024-01155-y},
issn = {2520-1158},
year = {2024},
date = {2024-05-15},
urldate = {2024-05-15},
journal = {Nature Catalysis},
volume = {7},
issue = {6},
pages = {666-677},
abstract = {Achieving the regioselective hydroformylation of linear α-olefins to linear aldehydes using solid catalysts with regioselectivities comparable to the corresponding homogeneous process is a great challenge in the chemical industry. Despite the tremendous efforts devoted to this research topic, most of the reported heterogeneous metal catalysts still give considerably lower regioselectivities than well-established homogeneous metal catalysts. Here we show the design of efficient Rh-zeolite catalysts, in which subnanometre Rh clusters are selectively confined in the sinusoidal ten-membered-ring channels of MFI zeolite, for the hydroformylation of long-chain linear α-olefins (C 6-C 12) into linear aldehydes with very high linear-to-branched aldehyde ratios (up to 400). The exceptional catalytic performances result from the involvement of the MFI zeolite framework as a rigid solid ligand that accommodates subnanometre Rh clusters in the sinusoidal channels of the MFI zeolite. Transition-metal-catalysed hydroformylation represents a premier route for the conversion of olefins into broadly applicable aldehydes and constitutes one of the largest homogeneous catalytic processes in the chemical industry (Fig. 1a) 1-3. Tremendous efforts have been devoted to the development of catalysts with high linear-to-branched (l/b) ratios of aldehydes because linear aldehydes and the derived alcohols are more desirable than the branched isomers in downstream processes (Fig. 1b) 4-7. Issues of unavoidable catalyst degradation , the necessary use of excessive/expensive ligands and catalyst separation/recycling impede further upgrading of the process and limit the adoption of olefins from different feedstock streams. In comparison with their homogeneous counterparts, heterogeneous catalysts are advantageous in terms of their separation/ recycling and their feasibility for implementation in continuous processes. Enduring efforts have been made to develop heterogeneous hydroformylation catalysts through multiple approaches (Fig. 1c) 8-15. However, none of these strategies can meet the high regioselectivity of the related homogeneous catalysts 16-19. To overcome the abovementioned limitations, we sought to investigate subnanometre Rh clusters confined in zeolites as tunable catalysts for olefin hydroformylation reactions, as the crystalline micropo-rous channels/cavities can not only accommodate the Rh sites with high structural uniformity but also provide a well-defined coordination environment for the Rh sites to constrain the transition states of the hydroformylation reaction for the selective production of linear aldehydes.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Achieving the regioselective hydroformylation of linear α-olefins to linear aldehydes using solid catalysts with regioselectivities comparable to the corresponding homogeneous process is a great challenge in the chemical industry. Despite the tremendous efforts devoted to this research topic, most of the reported heterogeneous metal catalysts still give considerably lower regioselectivities than well-established homogeneous metal catalysts. Here we show the design of efficient Rh-zeolite catalysts, in which subnanometre Rh clusters are selectively confined in the sinusoidal ten-membered-ring channels of MFI zeolite, for the hydroformylation of long-chain linear α-olefins (C 6-C 12) into linear aldehydes with very high linear-to-branched aldehyde ratios (up to 400). The exceptional catalytic performances result from the involvement of the MFI zeolite framework as a rigid solid ligand that accommodates subnanometre Rh clusters in the sinusoidal channels of the MFI zeolite. Transition-metal-catalysed hydroformylation represents a premier route for the conversion of olefins into broadly applicable aldehydes and constitutes one of the largest homogeneous catalytic processes in the chemical industry (Fig. 1a) 1-3. Tremendous efforts have been devoted to the development of catalysts with high linear-to-branched (l/b) ratios of aldehydes because linear aldehydes and the derived alcohols are more desirable than the branched isomers in downstream processes (Fig. 1b) 4-7. Issues of unavoidable catalyst degradation , the necessary use of excessive/expensive ligands and catalyst separation/recycling impede further upgrading of the process and limit the adoption of olefins from different feedstock streams. In comparison with their homogeneous counterparts, heterogeneous catalysts are advantageous in terms of their separation/ recycling and their feasibility for implementation in continuous processes. Enduring efforts have been made to develop heterogeneous hydroformylation catalysts through multiple approaches (Fig. 1c) 8-15. However, none of these strategies can meet the high regioselectivity of the related homogeneous catalysts 16-19. To overcome the abovementioned limitations, we sought to investigate subnanometre Rh clusters confined in zeolites as tunable catalysts for olefin hydroformylation reactions, as the crystalline micropo-rous channels/cavities can not only accommodate the Rh sites with high structural uniformity but also provide a well-defined coordination environment for the Rh sites to constrain the transition states of the hydroformylation reaction for the selective production of linear aldehydes.8.Peng Yin, Xiangfu Niu, Shuo-bin Li, Kai Chen, Xi Zhang, Ming Zuo, Liang Zhang, Hai-Wei Liang, "Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts", Nature Communications, 15, 1, 2024, 415.
@article{nokey,
title = {Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts},
author = {Peng Yin and Xiangfu Niu and Shuo-bin Li and Kai Chen and Xi Zhang and Ming Zuo and Liang Zhang and Hai-Wei Liang},
url = {https://doi.org/10.1038/s41467-023-44674-1},
doi = {10.1038/s41467-023-44674-1},
issn = {2041-1723},
year = {2024},
date = {2024-01-10},
urldate = {2024-01-10},
journal = {Nature Communications},
volume = {15},
issue = {1},
pages = {415},
abstract = {Carbon supported PtCo intermetallic alloys are known to be one of the most promising candidates as low-platinum oxygen reduction reaction electrocatalysts for proton-exchange-membrane fuel cells. Nevertheless, the intrinsic trade-off between particle size and ordering degree of PtCo makes it challenging to simultaneously achieve a high specific activity and a large active surface area. Here, by machine-learning-accelerated screenings from the immense configuration space, we are able to statistically quantify the impact of chemical ordering on thermodynamic stability. We find that introducing of Cu/Ni into PtCo can provide additional stabilization energy by inducing Co-Cu/Ni disorder, thus facilitating the ordering process and achieveing an improved tradeoff between specific activity and active surface area. Guided by the theoretical prediction, the small sized and highly ordered ternary Pt2CoCu and Pt2CoNi catalysts are experimentally prepared, showing a large electrochemically active surface area of ~90 m2 gPt‒1 and a high specific activity of ~3.5 mA cm‒2.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Carbon supported PtCo intermetallic alloys are known to be one of the most promising candidates as low-platinum oxygen reduction reaction electrocatalysts for proton-exchange-membrane fuel cells. Nevertheless, the intrinsic trade-off between particle size and ordering degree of PtCo makes it challenging to simultaneously achieve a high specific activity and a large active surface area. Here, by machine-learning-accelerated screenings from the immense configuration space, we are able to statistically quantify the impact of chemical ordering on thermodynamic stability. We find that introducing of Cu/Ni into PtCo can provide additional stabilization energy by inducing Co-Cu/Ni disorder, thus facilitating the ordering process and achieveing an improved tradeoff between specific activity and active surface area. Guided by the theoretical prediction, the small sized and highly ordered ternary Pt2CoCu and Pt2CoNi catalysts are experimentally prepared, showing a large electrochemically active surface area of ~90 m2 gPt‒1 and a high specific activity of ~3.5 mA cm‒2.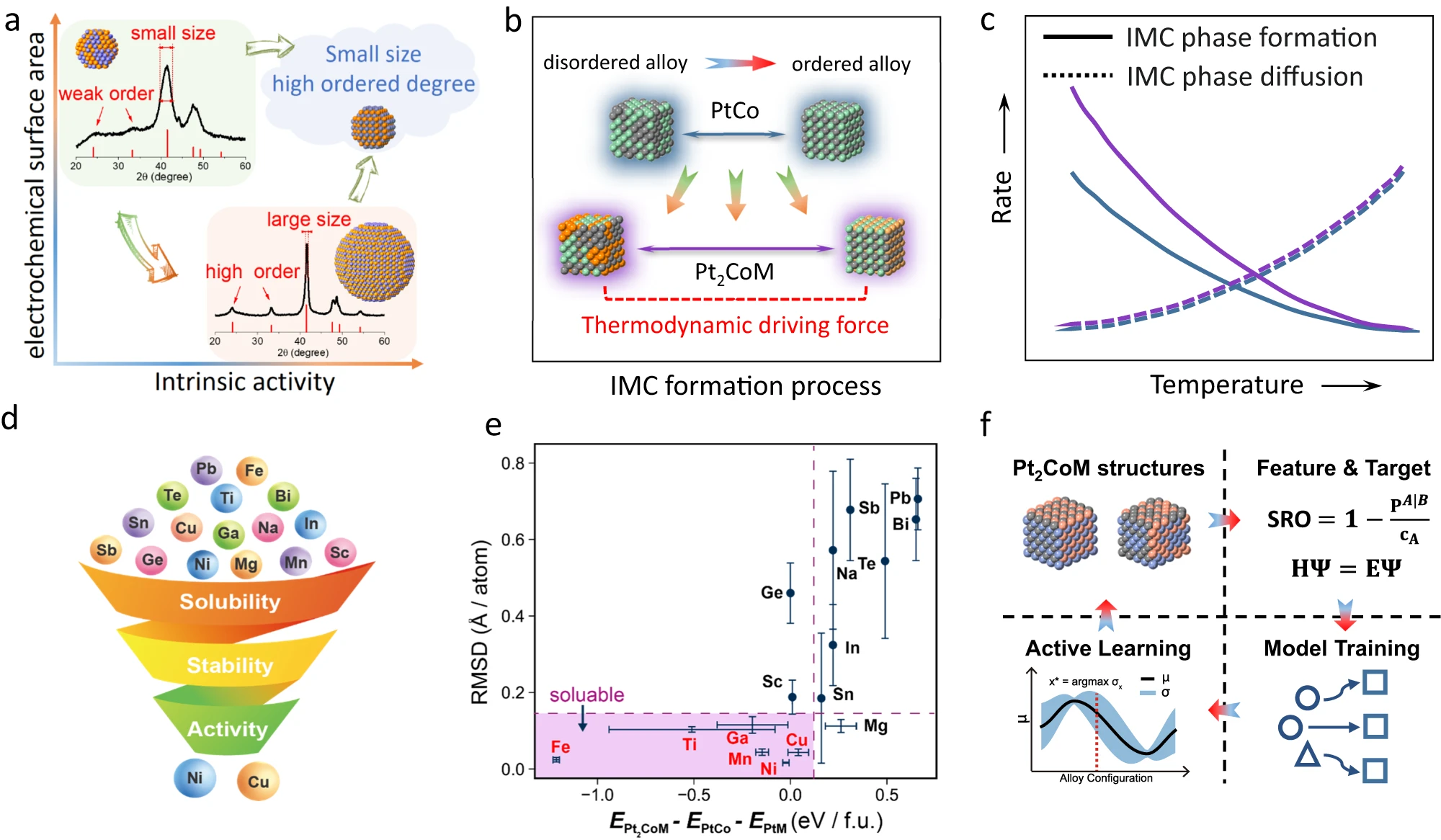 9.
9.Tang, Tang, Liu, XiaoZhi, Luo, Xuan, Xue, Zhuangzhuang, Pan, Hai-Rui, Fu, Jiaju, Yao, Ze-Cheng, Jiang, Zhe, Lyu, Zhen-Hua, Zheng, Lirong, Su, Dong, Zhang, Jia-Nan, Zhang, Liang, Hu, Jin-Song, "Unconventional Bilateral Compressive Strained Ni–Ir Interface Synergistically Accelerates Alkaline Hydrogen Oxidation", Journal of the American Chemical Society, 145, 25, 2023, 13805–13815.
@article{doi:10.1021/jacs.3c02487,
title = {Unconventional Bilateral Compressive Strained Ni–Ir Interface Synergistically Accelerates Alkaline Hydrogen Oxidation},
author = {Tang, Tang and Liu, XiaoZhi and Luo, Xuan and Xue, Zhuangzhuang and Pan, Hai-Rui and Fu, Jiaju and Yao, Ze-Cheng and Jiang, Zhe and Lyu, Zhen-Hua and Zheng, Lirong and Su, Dong and Zhang, Jia-Nan and Zhang, Liang and Hu, Jin-Song},
url = {https://doi.org/10.1021/jacs.3c02487},
doi = {10.1021/jacs.3c02487},
year = {2023},
date = {2023-06-28},
urldate = {2023-06-28},
journal = {Journal of the American Chemical Society},
volume = {145},
number = {25},
pages = {13805–13815},
abstract = {The alkaline hydrogen oxidation reaction (HOR) involves the coupling of adsorbed hydrogen (Had) and hydroxyl (OHad) species and is thus orders of magnitude slower than that in acid media. According to the Sabatier principle, developing electrocatalysts with appropriate binding energy for both intermediates is vital to accelerating the HOR though it is still challenging. Herein, we propose an unconventional bilateral compressive strained Ni–Ir interface (Ni–Ir(BCS)) as efficient synergistic HOR sites. Density functional theory (DFT) simulations reveal that the bilateral compressive strain effect leads to the appropriate adsorption for both Had and OHad, enabling their coupling thermodynamically spontaneous and kinetically preferential. Such Ni–Ir(BCS) is experimentally achieved by embedding sub-nanometer Ir clusters in graphene-loaded high-density Ni nanocrystals (Ni–Ir(BCS)/G). As predicted, it exhibits a HOR mass activity of 7.95 and 2.88 times those of commercial Ir/C and Pt/C together with much enhanced CO tolerance, respectively, ranking among the most active state-of-the-art HOR catalysts. These results provide new insights into the rational design of advanced electrocatalysts involving coordinated adsorption and activation of multiple reactants.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The alkaline hydrogen oxidation reaction (HOR) involves the coupling of adsorbed hydrogen (Had) and hydroxyl (OHad) species and is thus orders of magnitude slower than that in acid media. According to the Sabatier principle, developing electrocatalysts with appropriate binding energy for both intermediates is vital to accelerating the HOR though it is still challenging. Herein, we propose an unconventional bilateral compressive strained Ni–Ir interface (Ni–Ir(BCS)) as efficient synergistic HOR sites. Density functional theory (DFT) simulations reveal that the bilateral compressive strain effect leads to the appropriate adsorption for both Had and OHad, enabling their coupling thermodynamically spontaneous and kinetically preferential. Such Ni–Ir(BCS) is experimentally achieved by embedding sub-nanometer Ir clusters in graphene-loaded high-density Ni nanocrystals (Ni–Ir(BCS)/G). As predicted, it exhibits a HOR mass activity of 7.95 and 2.88 times those of commercial Ir/C and Pt/C together with much enhanced CO tolerance, respectively, ranking among the most active state-of-the-art HOR catalysts. These results provide new insights into the rational design of advanced electrocatalysts involving coordinated adsorption and activation of multiple reactants. 10.
10.Xin Geng, Shuwei Li, Lalani Mawella-Vithanage, Tao Ma, Mohamed Kilani, Bingwen Wang, Lu Ma, Chathuranga C Hewa-Rahinduwage, Alina Shafikova, Eranda Nikolla, Guangzhao Mao, Stephanie L. Brock, Liang Zhang, Long Luo, "Atomically dispersed Pb ionic sites in PbCdSe quantum dot gels enhance room-temperature NO2 sensing", Nature communications, 12, 1, 2021, 1-11.
@article{geng2021atomically,
title = {Atomically dispersed Pb ionic sites in PbCdSe quantum dot gels enhance room-temperature NO2 sensing},
author = {Xin Geng and Shuwei Li and Lalani Mawella-Vithanage and Tao Ma and Mohamed Kilani and Bingwen Wang and Lu Ma and Chathuranga C Hewa-Rahinduwage and Alina Shafikova and Eranda Nikolla and Guangzhao Mao and Stephanie L. Brock and Liang Zhang and Long Luo},
url = {https://www.nature.com/articles/s41467-021-25192-4.pdf, PDF},
doi = {10.1038/s41467-021-25192-4},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Nature communications},
volume = {12},
number = {1},
pages = {1-11},
publisher = {Nature Publishing Group},
abstract = {Atmospheric NO2 is of great concern due to its adverse effects on human health and the environment, motivating research on NO2 detection and remediation. Existing low-cost room-temperature NO2 sensors often suffer from low sensitivity at the ppb level or long recovery times, reflecting the trade-off between sensor response and recovery time. Here, we report an atomically dispersed metal ion strategy to address it. We discover that bimetallic PbCdSe quantum dot (QD) gels containing atomically dispersed Pb ionic sites achieve the optimal combination of strong sensor response and fast recovery, leading to a high-performance room-temperature p-type semiconductor NO2 sensor as characterized by a combination of ultra–low limit of detection, high sensitivity and stability, fast response and recovery. With the help of theoretical calculations, we reveal the high performance of the PbCdSe QD gel arises from the unique tuning effects of Pb ionic sites on NO2 binding at their neighboring Cd sites.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Atmospheric NO2 is of great concern due to its adverse effects on human health and the environment, motivating research on NO2 detection and remediation. Existing low-cost room-temperature NO2 sensors often suffer from low sensitivity at the ppb level or long recovery times, reflecting the trade-off between sensor response and recovery time. Here, we report an atomically dispersed metal ion strategy to address it. We discover that bimetallic PbCdSe quantum dot (QD) gels containing atomically dispersed Pb ionic sites achieve the optimal combination of strong sensor response and fast recovery, leading to a high-performance room-temperature p-type semiconductor NO2 sensor as characterized by a combination of ultra–low limit of detection, high sensitivity and stability, fast response and recovery. With the help of theoretical calculations, we reveal the high performance of the PbCdSe QD gel arises from the unique tuning effects of Pb ionic sites on NO2 binding at their neighboring Cd sites.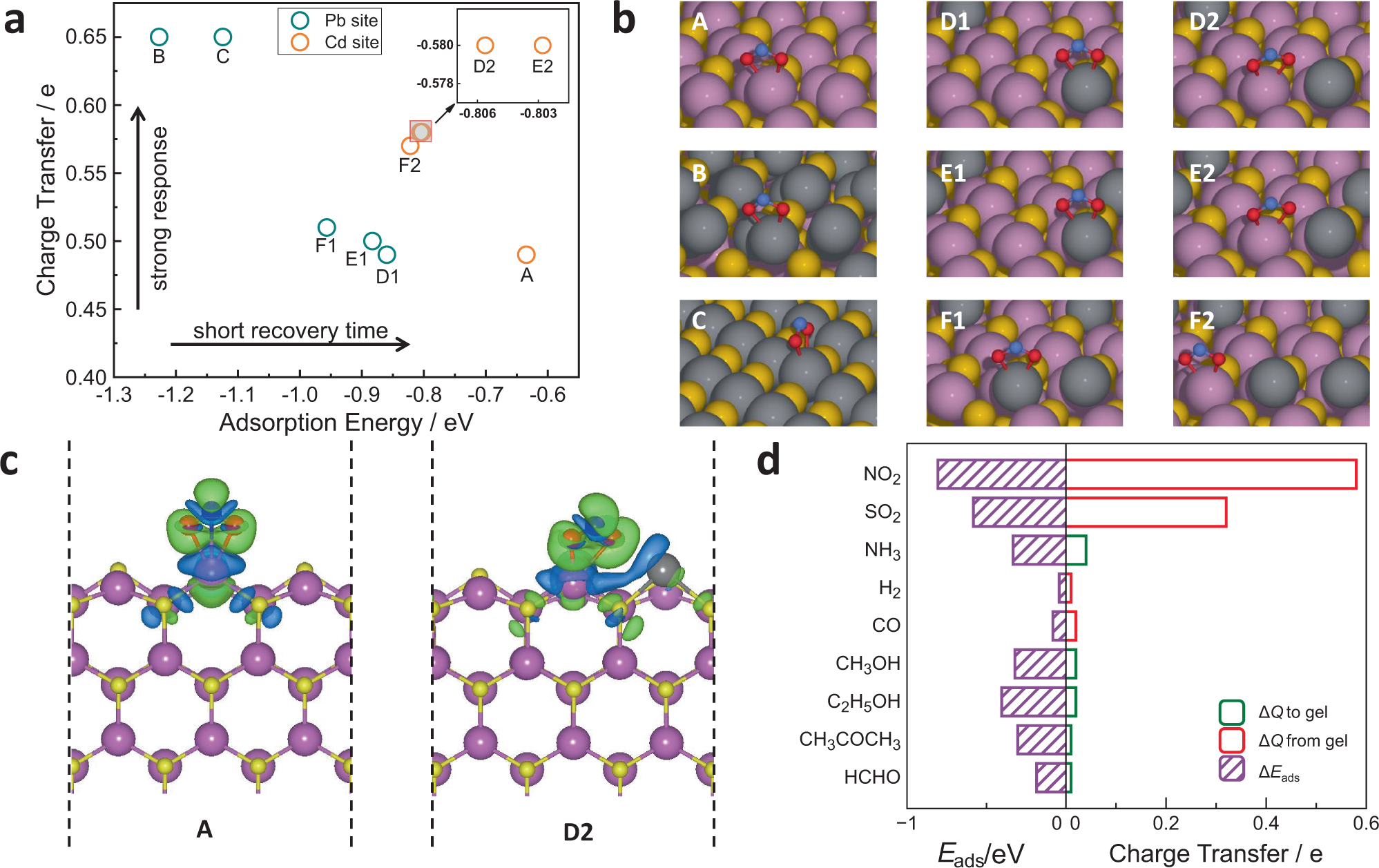 11.
11.Liang Zhang, May Ling Ng, Aleksandra Vojvodic, "Role of Undercoordinated Sites for the Catalysis in Confined Spaces Formed by Two-Dimensional Material Overlayers", The Journal of Physical Chemistry Letters, 11, 21, 2020, 9400-9407.
@article{Zhang2020bb,
title = {Role of Undercoordinated Sites for the Catalysis in Confined Spaces Formed by Two-Dimensional Material Overlayers},
author = {Liang Zhang and May Ling Ng and Aleksandra Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.jpclett.0c02652.pdf, PDF},
doi = {10.1021/acs.jpclett.0c02652},
issn = {1948-7185},
year = {2020},
date = {2020-11-01},
urldate = {2020-11-01},
journal = {The Journal of Physical Chemistry Letters},
volume = {11},
number = {21},
pages = {9400--9407},
abstract = {Adding a two-dimensional (2D) overlayer on a metal surface is a promising route for activating reactants confined in the interfacial space. However, an atomistic understanding of the role played by undercoordinated sites of the 2D overlayer in the activation of molecules in this nanoscaled confined space is yet to be developed. In this paper, we study CO dissociation as a prototypical reaction to investigate CO activation in the confined space enclosed by Rh(111) and a monolayer of hexagonal boron nitride (h-BN). The effect of the space size (i.e., the distance between h-BN and the metal surface), the type of undercoordinated sites, and the size of the defect are explicitly studied by density functional theory with dispersion correction. The following temperature-programmed X-ray photoelectron spectroscopy measurement suggests that a small portion of the CO dissociated during the desorption, leaving the residual atomic oxygen incorporated into the h-BN lattice, which validates the theoretical prediction. The combination of theory and experiment calls for further attention to be paid to the role of undercoordinated sites in the 2D overlayers in confined systems forming potential new catalytic environments.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Adding a two-dimensional (2D) overlayer on a metal surface is a promising route for activating reactants confined in the interfacial space. However, an atomistic understanding of the role played by undercoordinated sites of the 2D overlayer in the activation of molecules in this nanoscaled confined space is yet to be developed. In this paper, we study CO dissociation as a prototypical reaction to investigate CO activation in the confined space enclosed by Rh(111) and a monolayer of hexagonal boron nitride (h-BN). The effect of the space size (i.e., the distance between h-BN and the metal surface), the type of undercoordinated sites, and the size of the defect are explicitly studied by density functional theory with dispersion correction. The following temperature-programmed X-ray photoelectron spectroscopy measurement suggests that a small portion of the CO dissociated during the desorption, leaving the residual atomic oxygen incorporated into the h-BN lattice, which validates the theoretical prediction. The combination of theory and experiment calls for further attention to be paid to the role of undercoordinated sites in the 2D overlayers in confined systems forming potential new catalytic environments. 12.
12.Liang Zhang, Abhinav S Raman, Aleksandra Vojvodic, "Reviving Inert Oxides for Electrochemical Water Splitting by Subsurface Engineering", Chemistry of Materials, 32, 13, 2020, 5569-5578.
@article{Zhang2020a,
title = {Reviving Inert Oxides for Electrochemical Water Splitting by Subsurface Engineering},
author = {Liang Zhang and Abhinav S Raman and Aleksandra Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.chemmater.0c00763.pdf, PDF},
doi = {10.1021/acs.chemmater.0c00763},
issn = {0897-4756},
year = {2020},
date = {2020-07-01},
urldate = {2020-07-01},
journal = {Chemistry of Materials},
volume = {32},
number = {13},
pages = {5569--5578},
abstract = {Recently, it was theoretically predicted and experimentally validated that subsurface alloying of SrRuO3 (SRO) beneath the SrTiO3 (STO) capping layer can significantly promote the otherwise inert STO surface toward oxygen evolution [ Akbashev et al. Energy Environ. Sci. 2018, 11, 1762-1769[. Herein, we provide a generalized framework behind the concept of subsurface alloying with different transition-metal dopants, host metal oxides, and doping levels. Based on density functional theory (DFT) calculations and detailed electronic-structure analysis, we first identify the electronic structure origin of the activation and stabilization phenomena and propose a tuning mechanism that enables the identification of candidate subsurface dopants in STO, with the highest activity for both oxygen and hydrogen evolution reactions. We then show that the proposed mechanism is applicable to subsurface alloys formed with other host materials such as SrZrO3, TiO2, and ZrO2. Finally, we propose a materials design scheme using partial subsurface alloying for more precise tuning of surface reactivity and activity. By generalizing the concept of subsurface alloying of metal oxides, our work explains why the SRO subsurface alloyed STO has among the highest OER enhancements and importantly provides a new route in tailoring the activity and stability of earth-abundant electrocatalysts for water splitting.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Recently, it was theoretically predicted and experimentally validated that subsurface alloying of SrRuO3 (SRO) beneath the SrTiO3 (STO) capping layer can significantly promote the otherwise inert STO surface toward oxygen evolution [ Akbashev et al. Energy Environ. Sci. 2018, 11, 1762-1769[. Herein, we provide a generalized framework behind the concept of subsurface alloying with different transition-metal dopants, host metal oxides, and doping levels. Based on density functional theory (DFT) calculations and detailed electronic-structure analysis, we first identify the electronic structure origin of the activation and stabilization phenomena and propose a tuning mechanism that enables the identification of candidate subsurface dopants in STO, with the highest activity for both oxygen and hydrogen evolution reactions. We then show that the proposed mechanism is applicable to subsurface alloys formed with other host materials such as SrZrO3, TiO2, and ZrO2. Finally, we propose a materials design scheme using partial subsurface alloying for more precise tuning of surface reactivity and activity. By generalizing the concept of subsurface alloying of metal oxides, our work explains why the SRO subsurface alloyed STO has among the highest OER enhancements and importantly provides a new route in tailoring the activity and stability of earth-abundant electrocatalysts for water splitting. 13.
13.A R Akbashev, L Zhang, J T Mefford, J Park, B Butz, H Luftman, W C Chueh, A Vojvodic, "Activation of ultrathin SrTiO 3 with subsurface SrRuO 3 for the oxygen evolution reaction", Energy & Environmental Science, 11, 7, 2018, 1762-1769.
@article{Akbashev2018,
title = {Activation of ultrathin SrTiO 3 with subsurface SrRuO 3 for the oxygen evolution reaction},
author = {A R Akbashev and L Zhang and J T Mefford and J Park and B Butz and H Luftman and W C Chueh and A Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/c8ee00210j.pdf, PDF},
doi = {10.1039/C8EE00210J},
issn = {1754-5692},
year = {2018},
date = {2018-01-01},
urldate = {2018-01-01},
journal = {Energy & Environmental Science},
volume = {11},
number = {7},
pages = {1762--1769},
abstract = {Water electrolysis occurs via the oxygen/hydrogen evolution reactions. Achieving sufficiently high oxygen evolution reaction (OER) activity while maintaining stability of the active catalyst surface is a primary challenge of designing OER catalysts. Often high electrocatalytic activity is accompanied by structural instability. This is the case for the metallic perovskite oxide SrRuO3 (SRO), which exhibits rapid dissolution under OER conditions, both as thin-films and as nanoparticles. On the other hand, large band-gap perovskite oxides such as SrTiO3 (STO) are inactive for OER in the dark but are stable. In this work, we demonstrate that burying as little as one unit cell of SRO beneath an ultrathin STO capping layer activates the otherwise inert electrocatalyst and gives excellent stability. Using density functional theory (DFT) calculations, we find that such a chemical modification of STO by sub-surface SRO introduces new 4d electronic states including Ru states within the STO band gap, raising the energy level of the electrons, changing the electronic hybridization, and facilitating an easy transfer to the adsorbed intermediates. We validate this hypothesis experimentally using atomically precise heteroepitaxial deposition. We find that a single-unit-cell layer of SRO is sufficient to activate the topmost STO layer towards OER; burying SRO underneath two unit cells of STO protects the inherently unstable SRO against corrosion during OER. Generally, our layered heterostructures are model systems of oxide core–shell structures, where an unstable catalyst (core) is protected against degradation and electronically activates the otherwise inactive shell. As demonstrated in this work, the growth of ultrathin heterostructures establishes a rigorous platform for screening core/shell nanoparticle combinations/thicknesses, and elucidates sub-surface activation mechanism for achieving stable and active oxide electrocatalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Water electrolysis occurs via the oxygen/hydrogen evolution reactions. Achieving sufficiently high oxygen evolution reaction (OER) activity while maintaining stability of the active catalyst surface is a primary challenge of designing OER catalysts. Often high electrocatalytic activity is accompanied by structural instability. This is the case for the metallic perovskite oxide SrRuO3 (SRO), which exhibits rapid dissolution under OER conditions, both as thin-films and as nanoparticles. On the other hand, large band-gap perovskite oxides such as SrTiO3 (STO) are inactive for OER in the dark but are stable. In this work, we demonstrate that burying as little as one unit cell of SRO beneath an ultrathin STO capping layer activates the otherwise inert electrocatalyst and gives excellent stability. Using density functional theory (DFT) calculations, we find that such a chemical modification of STO by sub-surface SRO introduces new 4d electronic states including Ru states within the STO band gap, raising the energy level of the electrons, changing the electronic hybridization, and facilitating an easy transfer to the adsorbed intermediates. We validate this hypothesis experimentally using atomically precise heteroepitaxial deposition. We find that a single-unit-cell layer of SRO is sufficient to activate the topmost STO layer towards OER; burying SRO underneath two unit cells of STO protects the inherently unstable SRO against corrosion during OER. Generally, our layered heterostructures are model systems of oxide core–shell structures, where an unstable catalyst (core) is protected against degradation and electronically activates the otherwise inactive shell. As demonstrated in this work, the growth of ultrathin heterostructures establishes a rigorous platform for screening core/shell nanoparticle combinations/thicknesses, and elucidates sub-surface activation mechanism for achieving stable and active oxide electrocatalysts.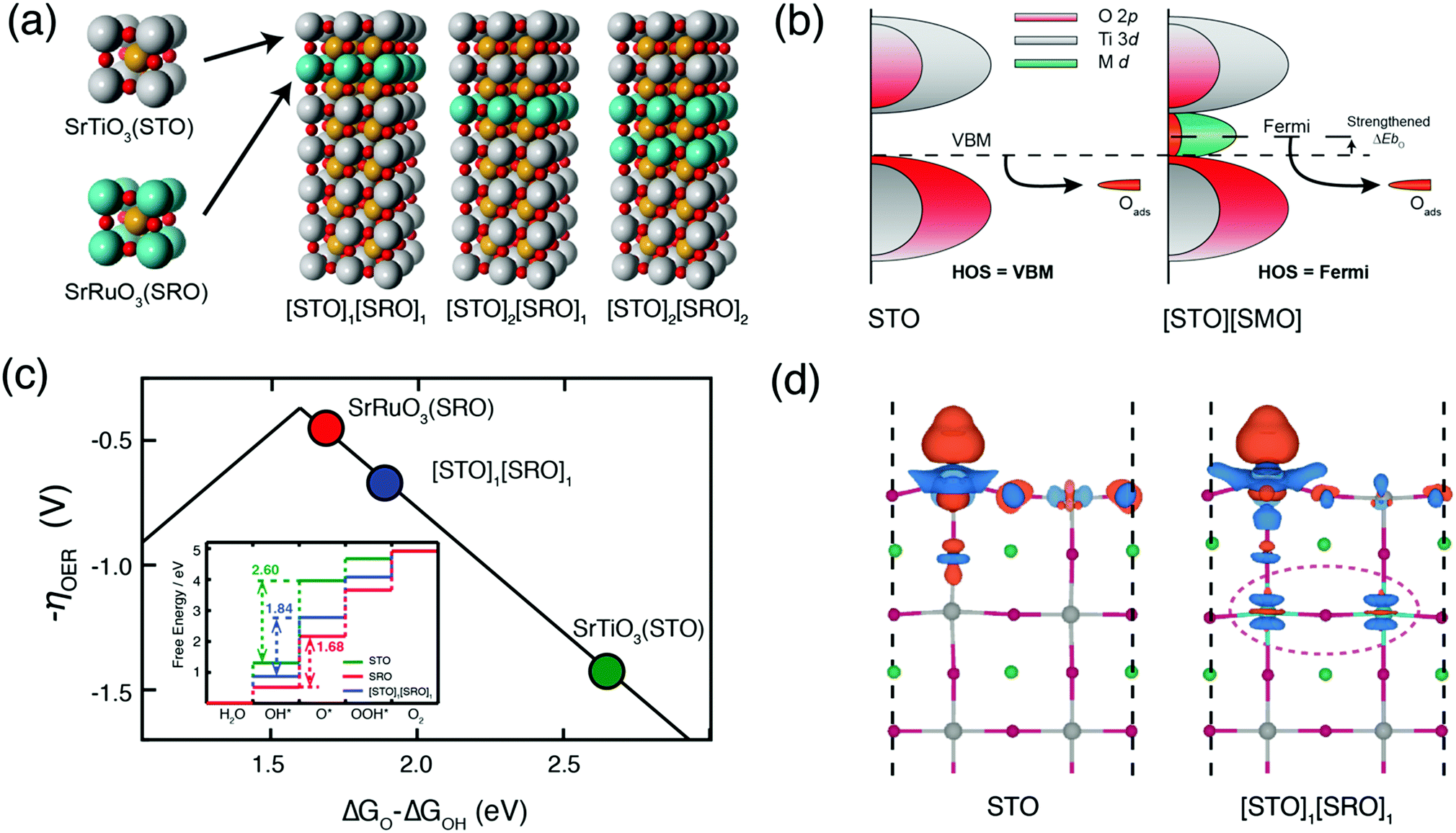 14.
14.Liang Zhang, Samuel T Chill, Graeme Henkelman, "Distributed replica dynamics", The Journal of Chemical Physics, 143, 17, 2015, 174112.
@article{Zhang2015c,
title = {Distributed replica dynamics},
author = {Liang Zhang and Samuel T Chill and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/JCP1.4934987.pdf, PDF},
doi = {10.1063/1.4934987},
issn = {0021-9606},
year = {2015},
date = {2015-11-01},
urldate = {2015-11-01},
journal = {The Journal of Chemical Physics},
volume = {143},
number = {17},
pages = {174112},
abstract = {A distributed replica dynamics (DRD) method is proposed to calculate rare-event molecular dynamics using distributed computational resources. Similar to Voter's parallel replica dynamics (PRD) method, the dynamics of independent replicas of the system are calculated on different computational clients. In DRD, each replica runs molecular dynamics from an initial state for a fixed simulation time and then reports information about the trajectory back to the server. A simulation clock on the server accumulates the simulation time of each replica until one reports a transition to a new state. Subsequent calculations are initiated from within this new state and the process is repeated to follow the state-to-state evolution of the system. DRD is designed to work with asynchronous and distributed computing resources in which the clients may not be able to communicate with each other. Additionally, clients can be added or removed from the simulation at any point in the calculation. Even with heterogeneous computing clients, we prove that the DRD method reproduces the correct probability distribution of escape times. We also show this correspondence numerically; molecular dynamics simulations of Al(100) adatom diffusion using PRD and DRD give consistent exponential distributions of escape times. Finally, we discuss guidelines for choosing the optimal number of replicas and replica trajectory length for the DRD method.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
A distributed replica dynamics (DRD) method is proposed to calculate rare-event molecular dynamics using distributed computational resources. Similar to Voter's parallel replica dynamics (PRD) method, the dynamics of independent replicas of the system are calculated on different computational clients. In DRD, each replica runs molecular dynamics from an initial state for a fixed simulation time and then reports information about the trajectory back to the server. A simulation clock on the server accumulates the simulation time of each replica until one reports a transition to a new state. Subsequent calculations are initiated from within this new state and the process is repeated to follow the state-to-state evolution of the system. DRD is designed to work with asynchronous and distributed computing resources in which the clients may not be able to communicate with each other. Additionally, clients can be added or removed from the simulation at any point in the calculation. Even with heterogeneous computing clients, we prove that the DRD method reproduces the correct probability distribution of escape times. We also show this correspondence numerically; molecular dynamics simulations of Al(100) adatom diffusion using PRD and DRD give consistent exponential distributions of escape times. Finally, we discuss guidelines for choosing the optimal number of replicas and replica trajectory length for the DRD method. 15.
15.Liang Zhang, Graeme Henkelman, "Computational Design of Alloy-Core@Shell Metal Nanoparticle Catalysts", ACS Catalysis, 5, 2, 2015, 655-660.
@article{Zhang2015b,
title = {Computational Design of Alloy-Core@Shell Metal Nanoparticle Catalysts},
author = {Liang Zhang and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/cs501176b.pdf, PDF},
doi = {10.1021/cs501176b},
issn = {2155-5435},
year = {2015},
date = {2015-02-01},
urldate = {2015-02-01},
journal = {ACS Catalysis},
volume = {5},
number = {2},
pages = {655--660},
abstract = {The alloy-core@shell nanoparticle structure combines the advantages of a robust noble-metal shell and a tunable alloy-core composition. In this study we demonstrate a set of linear correlations between the binding of adsorbates to the shell and the alloy-core composition, which are general across a range of nanoparticle compositions, size, and adsorbate molecules. This systematic tunability allows for a simple approach to the design of such catalysts. Calculations of candidate structures for the hydrogen evolution reaction predict a high activity for the PtRu@Pd structure, in good agreement with what has been reported previously. Calculations of alloy-core@Pt 140-atom nanoparticles reveal new candidate structures for CO oxidation at high temperature, including Au0.65Pd0.35@Pt and Au0.73Pt0.27@Pt, which are predicted to have reaction rates 200 times higher than that of Pt(111).},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The alloy-core@shell nanoparticle structure combines the advantages of a robust noble-metal shell and a tunable alloy-core composition. In this study we demonstrate a set of linear correlations between the binding of adsorbates to the shell and the alloy-core composition, which are general across a range of nanoparticle compositions, size, and adsorbate molecules. This systematic tunability allows for a simple approach to the design of such catalysts. Calculations of candidate structures for the hydrogen evolution reaction predict a high activity for the PtRu@Pd structure, in good agreement with what has been reported previously. Calculations of alloy-core@Pt 140-atom nanoparticles reveal new candidate structures for CO oxidation at high temperature, including Au0.65Pd0.35@Pt and Au0.73Pt0.27@Pt, which are predicted to have reaction rates 200 times higher than that of Pt(111).
Journal Articles
Years 2011 – 2024
- 1.
Yue Pan, Shiyu Zhen, Xiaozhi Liu, Mengshu Ge, Jianxiong Zhao, Lin Gu, Dan Zhou, Liang Zhang, Dong Su, "Looping metal-support interaction in heterogeneous catalysts during redox reactions", Nat Commun, 16, 8627, 2025.
 2.
2.Xiangfu Niu, Yanjun Chen, Mingze Sun, Satoshi Nagao, Yuki Aoki, Zhiqiang Niu, Liang Zhang, "Bayesian-Learning-Assisted Catalyst Discovery for Efficient Iridium Utilization in Electrochemical Water Splitting", Science Advances, 11, 34, 2025, eadw0894.
 3.
3.Xiangfu Niu, Shiyu Zhen, Rui Zhang, Jianqiu Li, Liang Zhang, "Unraveling Disorder-to-Order Transitions and Chemical Ordering in PtCoM Ternary Alloys Using Machine Learning Potential", Chemical Science , 16, 33, 2025, 14884-14893.
4.Xiangfu Niu, Shuwei Li, Zheyu Zhang, Haohong Duan, Rui Zhang, Jianqiu Li, Liang Zhang, "Accelerated Optimization of Compositions and Chemical Ordering for Bimetallic Alloy Catalysts Using Bayesian Learning", ACS Catalysis, 2025, 4374-4383.
 5.
5.Zhe He, Kailang Li, Tianxiang Chen, Yunchao Feng, Eduardo Villalobos-Portillo, Carlo Marini, Tsz Woon Benedict Lo, Fuyuan Yang, Liang Zhang, Lichen Liu, "High-purity hydrogen production from dehydrogenation of methylcyclohexane catalyzed by zeolite-encapsulated subnanometer platinum-iron clusters", Nature Communications, 16, 1, 2025, 92.
 6.
6.Shuwei Li, Liang Zhang, "Accurate first-principles simulation for the response of 2D chemiresistive gas sensors", npj Comput Mater, 10, 1, 2024.
 7.
7.Xiaomeng Dou, Tao Yan, Lixiang Qian, Huaming Hou, Miguel Lopez-Haro, Carlo Marini, Giovanni Agostini, Debora M Meira, Xiangjie Zhang, Liang Zhang, Zhi Cao, Lichen Liu, "Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite", Nature Catalysis, 7, 6, 2024, 666-677.
8.Peng Yin, Xiangfu Niu, Shuo-bin Li, Kai Chen, Xi Zhang, Ming Zuo, Liang Zhang, Hai-Wei Liang, "Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts", Nature Communications, 15, 1, 2024, 415.
 9.
9.Tang, Tang, Liu, XiaoZhi, Luo, Xuan, Xue, Zhuangzhuang, Pan, Hai-Rui, Fu, Jiaju, Yao, Ze-Cheng, Jiang, Zhe, Lyu, Zhen-Hua, Zheng, Lirong, Su, Dong, Zhang, Jia-Nan, Zhang, Liang, Hu, Jin-Song, "Unconventional Bilateral Compressive Strained Ni–Ir Interface Synergistically Accelerates Alkaline Hydrogen Oxidation", Journal of the American Chemical Society, 145, 25, 2023, 13805–13815.
 10.
10.Xin Geng, Shuwei Li, Lalani Mawella-Vithanage, Tao Ma, Mohamed Kilani, Bingwen Wang, Lu Ma, Chathuranga C Hewa-Rahinduwage, Alina Shafikova, Eranda Nikolla, Guangzhao Mao, Stephanie L. Brock, Liang Zhang, Long Luo, "Atomically dispersed Pb ionic sites in PbCdSe quantum dot gels enhance room-temperature NO2 sensing", Nature communications, 12, 1, 2021, 1-11.
 11.
11.Liang Zhang, May Ling Ng, Aleksandra Vojvodic, "Role of Undercoordinated Sites for the Catalysis in Confined Spaces Formed by Two-Dimensional Material Overlayers", The Journal of Physical Chemistry Letters, 11, 21, 2020, 9400-9407.
 12.
12.Liang Zhang, Abhinav S Raman, Aleksandra Vojvodic, "Reviving Inert Oxides for Electrochemical Water Splitting by Subsurface Engineering", Chemistry of Materials, 32, 13, 2020, 5569-5578.
 13.
13.A R Akbashev, L Zhang, J T Mefford, J Park, B Butz, H Luftman, W C Chueh, A Vojvodic, "Activation of ultrathin SrTiO 3 with subsurface SrRuO 3 for the oxygen evolution reaction", Energy & Environmental Science, 11, 7, 2018, 1762-1769.
 14.
14.Liang Zhang, Samuel T Chill, Graeme Henkelman, "Distributed replica dynamics", The Journal of Chemical Physics, 143, 17, 2015, 174112.
 15.
15.Liang Zhang, Graeme Henkelman, "Computational Design of Alloy-Core@Shell Metal Nanoparticle Catalysts", ACS Catalysis, 5, 2, 2015, 655-660.

2025
Yue Pan, Shiyu Zhen, Xiaozhi Liu, Mengshu Ge, Jianxiong Zhao, Lin Gu, Dan Zhou, Liang Zhang, Dong Su, "Looping metal-support interaction in heterogeneous catalysts during redox reactions", Nat Commun, 16, 8627, 2025.
@article{Pan2025b,
title = {Looping metal-support interaction in heterogeneous catalysts during redox reactions},
author = {Yue Pan and Shiyu Zhen and Xiaozhi Liu and Mengshu Ge and Jianxiong Zhao and Lin Gu and Dan Zhou and Liang Zhang and Dong Su},
doi = {10.1038/s41467-025-63646-1},
issn = {2041-1723},
year = {2025},
date = {2025-12-00},
urldate = {2025-12-00},
journal = {Nat Commun},
volume = {16},
number = {8627},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Haiyang Lan, Zixuan Xue, Jiakun Sun, Jiaming Chu, Jiangyuan Feng, Weitao Jin, Zixian Wang, Ruiqing Song, Pengfei Yan, Liang Zhang, Yucun Zhou, Xiaofeng Ye, Juan Zhou, "An in-situ generated multiphase catalyst for active and durable ammonia protonic ceramic fuel cells", Chemical Engineering Journal, 523, 168483, 2025.
@article{Lan2025,
title = {An in-situ generated multiphase catalyst for active and durable ammonia protonic ceramic fuel cells},
author = {Haiyang Lan and Zixuan Xue and Jiakun Sun and Jiaming Chu and Jiangyuan Feng and Weitao Jin and Zixian Wang and Ruiqing Song and Pengfei Yan and Liang Zhang and Yucun Zhou and Xiaofeng Ye and Juan Zhou},
doi = {10.1016/j.cej.2025.168483},
issn = {1385-8947},
year = {2025},
date = {2025-11-01},
urldate = {2025-11-01},
journal = {Chemical Engineering Journal},
volume = {523},
number = {168483},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Hongyang Su, Jing Chai, Zixuan Guan, Hendrik Bluhm, Ziyun Zhang, Yidan Cao, Zhi Liu, Yuan-Hua Lin, William C. Chueh, Liang Zhang, Di Chen, "Tuning the f Band for Enhanced Surface Redox in Strained Rare Earth Oxides", J. Am. Chem. Soc., 2025.
@article{Su2025,
title = {Tuning the f Band for Enhanced Surface Redox in Strained Rare Earth Oxides},
author = {Hongyang Su and Jing Chai and Zixuan Guan and Hendrik Bluhm and Ziyun Zhang and Yidan Cao and Zhi Liu and Yuan-Hua Lin and William C. Chueh and Liang Zhang and Di Chen},
doi = {10.1021/jacs.5c08872},
issn = {1520-5126},
year = {2025},
date = {2025-10-24},
urldate = {2025-10-24},
journal = {J. Am. Chem. Soc.},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Shiyu Zhen, Yong Wang, Yuhan Wang, Jianhao Wang, Xiangfu Niu, Kailang Li, Dong Su, Haohong Duan, Baorui Jia, Mingli Qin, Liang Zhang, "Machine-learning guided design of non-precious-metal high-entropy electrocatalysts for alkaline hydrogen evolution", eScience, 2025, 100484.
@article{nokey,
title = {Machine-learning guided design of non-precious-metal high-entropy electrocatalysts for alkaline hydrogen evolution},
author = {Shiyu Zhen, Yong Wang, Yuhan Wang, Jianhao Wang, Xiangfu Niu, Kailang Li, Dong Su, Haohong Duan, Baorui Jia, Mingli Qin and Liang Zhang},
doi = {10.1016/j.esci.2025.100484},
year = {2025},
date = {2025-10-20},
urldate = {2025-12-29},
journal = {eScience},
pages = {100484},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Jing Chai, Lixiang Qian, Yucun Zhou, Jianqiu Li, Liang Zhang, "The Direct Ammonia Oxidation Reaction Mechanism and Selectivity on Ni-YSZ Anodes in Solid Oxide Fuel Cells", ACS Catalysis, 2025, 18167–18176.
@article{nokeyf,
title = {The Direct Ammonia Oxidation Reaction Mechanism and Selectivity on Ni-YSZ Anodes in Solid Oxide Fuel Cells},
author = {Jing Chai, Lixiang Qian, Yucun Zhou, Jianqiu Li, Liang Zhang},
doi = {10.1021/acscatal.5c05016},
year = {2025},
date = {2025-10-18},
urldate = {2025-12-30},
journal = {ACS Catalysis},
pages = {18167–18176},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiangfu Niu, Yanjun Chen, Mingze Sun, Satoshi Nagao, Yuki Aoki, Zhiqiang Niu, Liang Zhang, "Bayesian-Learning-Assisted Catalyst Discovery for Efficient Iridium Utilization in Electrochemical Water Splitting", Science Advances, 11, 34, 2025, eadw0894.
@article{nokey,
title = {Bayesian-Learning-Assisted Catalyst Discovery for Efficient Iridium Utilization in Electrochemical Water Splitting},
author = {Xiangfu Niu, Yanjun Chen, Mingze Sun, Satoshi Nagao, Yuki Aoki, Zhiqiang Niu, Liang Zhang},
url = {https://www.science.org/doi/10.1126/sciadv.adw0894},
year = {2025},
date = {2025-08-20},
urldate = {2025-08-20},
journal = {Science Advances},
volume = {11},
issue = {34},
pages = {eadw0894},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Shuzhen Li, Xuan Luo, Yueshuai Wang, Chaowei Wang, Guizhen Zhang, Huixin Xiang, Yong Yan, Xiaoxing Ke, Yue Lu, Chuanhao Yao, Hongyi Li, Liang Zhang, Ge Chen, "Strong Oxide-Support Interaction Induced Thermal Stabilization of Pt Single Atoms for Durable Catalytic CO Oxidation", Angewandte Chemie International Edition, e202504551, 2025, e202504551.
@article{https://doi.org/10.1002/anie.202504551,
title = {Strong Oxide-Support Interaction Induced Thermal Stabilization of Pt Single Atoms for Durable Catalytic CO Oxidation},
author = {Shuzhen Li and Xuan Luo and Yueshuai Wang and Chaowei Wang and Guizhen Zhang and Huixin Xiang and Yong Yan and Xiaoxing Ke and Yue Lu and Chuanhao Yao and Hongyi Li and Liang Zhang and Ge Chen},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202504551},
doi = {https://doi.org/10.1002/anie.202504551},
year = {2025},
date = {2025-08-11},
urldate = {2025-08-11},
journal = {Angewandte Chemie International Edition},
volume = {n/a},
number = {e202504551},
pages = {e202504551},
abstract = {Abstract Supported metal nanoparticle catalysts often suffer from sintering-induced size-dependent deactivation, limiting their high-temperature applications. Although high-temperature redispersion offers a potential solution, this strategy remains restricted to reducible support materials, severely limiting the selection of catalyst supports with versatile compositions and tunable functionalities. Here, we engineer cationic vacancies at Al2O3-La2O3 interface via strong oxide-support interaction (SOSI)—driven interfacial reconstruction during calcination. The vacancy-mediated confinement effect dynamically intercepts migrating Pt species, enabling the construction of Al2O3-Pt1-La2O3 structure with precisely defined coordination environments. The resulting catalyst achieves complete CO conversion at 145 °C and maintains stability with minimal decline after a 6-h treatment at 1100 °C in air with 10% steam. This interfacial engineering strategy proves universal, as demonstrated by ZrO2-La2O3 counterparts. Our findings break the reducibility dependency in traditional single-atom catalysts (SACs) stabilization by establishing oxide–oxide interface as universal anchoring platforms, which expands the design space of industrial-grade SACs beyond conventional reducible oxides.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Wenxia Chen, Zhiyi Sun, Shiyu Zhen, Yujie Wang, Jingqi Sun, Meng Liu, Wen‐Jun Han, Lanmei Lai, Wei Wei, Liang Zhang, Wenxing Chen, "Tuning Asymmetric S‐Bridged Cu─Co Dual Sites at Atomic‐Level for Efficient Ammonia Electrosynthesis", Adv Funct Materials, 2025.
@article{Chen2025b,
title = {Tuning Asymmetric S‐Bridged Cu─Co Dual Sites at Atomic‐Level for Efficient Ammonia Electrosynthesis},
author = {Wenxia Chen and Zhiyi Sun and Shiyu Zhen and Yujie Wang and Jingqi Sun and Meng Liu and Wen‐Jun Han and Lanmei Lai and Wei Wei and Liang Zhang and Wenxing Chen},
doi = {10.1002/adfm.202509200},
issn = {1616-3028},
year = {2025},
date = {2025-08-05},
urldate = {2025-08-05},
journal = {Adv Funct Materials},
publisher = {Wiley},
abstract = {<jats:title>Abstract</jats:title><jats:p>Electrochemical nitrate reduction reaction (NO<jats:sub>3</jats:sub>RR) for ammonia production holds immense promise as an environmentally benign strategy. Nevertheless, the process faces inherent limitations by sluggish kinetics of the eight‐electron transfer and multiple competing reactions. Here, an asymmetric S‐bridged Cu, Co dual‐atom (Cu─S─Co) catalyst (CuCo‐SNC) is creatively reported by leveraging the abundant disulfide bond capture and chelation capabilities of wool keratin. Benefiting from the charge regulation effect between the metal sites and S‐bridged atoms, the CuCo‐SNC catalyst exhibits an optimal Faradaic efficiency of 97.8% at −0.3 V (vs RHE) and a remarkable NH<jats:sub>3</jats:sub> yield rate of 0.88 mmol h<jats:sup>−1</jats:sup> cm<jats:sup>−2</jats:sup> at −0.6 V (vs RHE). The assembled Zn‐NO<jats:sub>3</jats:sub><jats:sup>−</jats:sup> battery shows a power density of 7.99 mW cm<jats:sup>−2</jats:sup> and exceptional cycling stability. Furthermore, in situ characterization and theoretical analysis reveal that the S‐bridge breaks the electron balance between Cu and Co, effectively modulating the charge state of the Cu─Co site, which boosts electrons transfer from Cu to Co site through the S‐bridge, thereby promoting the efficient conversion of nitrate ions (NO<jats:sub>3</jats:sub><jats:sup>−</jats:sup>) at Co─Cu bimetallic sites. This asymmetric dual atom catalyst provides a novel perspective for the development of advanced electrocatalytic technologies in ammonia synthesis.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Cunyuan Gao, Yutong Wang, Shiyu Zhen, Jinhua Zhan, Liang Zhang, Bin Cai, "Rapid Synthesis of Carbon-Supported Ir–Ni Bimetallic Nanoparticles for Efficient Water Oxidation", J. Phys. Chem. C, 129, 28, 2025, 12796-12803.
@article{Gao2025b,
title = {Rapid Synthesis of Carbon-Supported Ir–Ni Bimetallic Nanoparticles for Efficient Water Oxidation},
author = {Cunyuan Gao and Yutong Wang and Shiyu Zhen and Jinhua Zhan and Liang Zhang and Bin Cai},
doi = {10.1021/acs.jpcc.5c03813},
issn = {1932-7455},
year = {2025},
date = {2025-07-17},
urldate = {2025-07-17},
journal = {J. Phys. Chem. C},
volume = {129},
number = {28},
pages = {12796--12803},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiangfu Niu, Shiyu Zhen, Rui Zhang, Jianqiu Li, Liang Zhang, "Unraveling Disorder-to-Order Transitions and Chemical Ordering in PtCoM Ternary Alloys Using Machine Learning Potential", Chemical Science , 16, 33, 2025, 14884-14893.
@article{nokey,
title = {Unraveling Disorder-to-Order Transitions and Chemical Ordering in PtCoM Ternary Alloys Using Machine Learning Potential},
author = {Xiangfu Niu, Shiyu Zhen, Rui Zhang, Jianqiu Li, Liang Zhang},
url = {https://doi.org/10.1039/D5SC04043D},
year = {2025},
date = {2025-07-15},
urldate = {2025-07-15},
journal = {Chemical Science },
volume = {16},
issue = {33},
pages = {14884-14893},
note = {selected},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Kai Chen, Lixiang Qian, Haohong Duan, Rui Zhang, Jianqiu Li, Liang Zhang, "Impact of surface oxygen functionalization on local oxygen transport resistance at the fuel cell cathode", Chemical Engineering Science, 313, 2025, 121745.
@article{Chen2025,
title = {Impact of surface oxygen functionalization on local oxygen transport resistance at the fuel cell cathode},
author = {Kai Chen and Lixiang Qian and Haohong Duan and Rui Zhang and Jianqiu Li and Liang Zhang},
url = {https://www.sciencedirect.com/science/article/pii/S0009250925005688},
issn = {0009-2509},
year = {2025},
date = {2025-07-01},
urldate = {2025-07-01},
journal = {Chemical Engineering Science},
volume = {313},
pages = {121745},
abstract = {The high oxygen transport resistance at the carbon/ionomer interface is one of the primary limitations for proton exchange membrane fuel cells (PEMFCs) with low platinum (Pt) loadings. This study uses molecular dynamics (MD) simulations to explore how oxygen-containing functional groups on carbon supports influence ionomer distribution and oxygen permeation. Results show that C=O and C-O-C groups prevent the dense overlay of ionomer backbones, enhancing phase segregation and improving both oxygen diffusion and solubility. By optimizing surface oxygen species and ratios (4 %–8%), these groups significantly reduce local oxygen transport resistance (RLocal). This work offers new insights for designing modified carbon supports to improve PEMFC performance by enhancing oxygen permeation and reducing RLocal.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xue Jia, Tianyi Wang, Di Zhang, Xuan Wang, Heng Liu, Liang Zhang, Hao Li, "Advancing electrocatalyst discovery through the lens of data science: State of the art and perspectives", Journal of Catalysis, 447, 2025, 116162.
@article{Jia2025,
title = {Advancing electrocatalyst discovery through the lens of data science: State of the art and perspectives},
author = {Xue Jia and Tianyi Wang and Di Zhang and Xuan Wang and Heng Liu and Liang Zhang and Hao Li},
doi = {10.1016/j.jcat.2025.116162},
issn = {0021-9517},
year = {2025},
date = {2025-07-00},
urldate = {2025-07-00},
journal = {Journal of Catalysis},
volume = {447},
pages = {116162},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Hongtao Wang, Kailang Li, Miguel Lopez-Haro, Carlo Marini, Zhe He, Minghao Gao, Liang Zhang, Lichen Liu, "Subnanometer Pt Catalysts Encapsulated in MEL Zeolite Mesocrystals for H2 Production from Methylcyclohexane Dehydrogenation", Journal of the American Chemical Society, 2025.
@article{Wang2025b,
title = {Subnanometer Pt Catalysts Encapsulated in MEL Zeolite Mesocrystals for H2 Production from Methylcyclohexane Dehydrogenation},
author = {Hongtao Wang and Kailang Li and Miguel Lopez-Haro and Carlo Marini and Zhe He and Minghao Gao and Liang Zhang and Lichen Liu},
url = {https://doi.org/10.1021/jacs.5c04618},
doi = {10.1021/jacs.5c04618},
issn = {0002-7863},
year = {2025},
date = {2025-06-11},
urldate = {2025-06-11},
journal = {Journal of the American Chemical Society},
publisher = {American Chemical Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Helai Huang, Mingze Sun, Kai Chen, Yizhen Che, Xin Tang, Zhengwen Li, Kaiqi Nie, Shuairen Qian, Jinjie Fang, Haiyong Wang, Yanfen Wu, Qikun Hu, Yuqi Wang, Xiaohang Sun, Junliang He, Yu-Xiao Zhang, Zhongbin Zhuang, Liang Zhang, Zhiqiang Niu, "Unlocking the Potential of Mn‐based Catalyst for Durable Two‐electron Oxygen Reduction in Acid at High Current Densities", Angew Chem Int Ed, 2025.
@article{Huang2025,
title = {Unlocking the Potential of Mn‐based Catalyst for Durable Two‐electron Oxygen Reduction in Acid at High Current Densities},
author = {Helai Huang and Mingze Sun and Kai Chen and Yizhen Che and Xin Tang and Zhengwen Li and Kaiqi Nie and Shuairen Qian and Jinjie Fang and Haiyong Wang and Yanfen Wu and Qikun Hu and Yuqi Wang and Xiaohang Sun and Junliang He and Yu-Xiao Zhang and Zhongbin Zhuang and Liang Zhang and Zhiqiang Niu},
doi = {10.1002/anie.202511844},
issn = {1521-3773},
year = {2025},
date = {2025-06-10},
urldate = {2025-06-10},
journal = {Angew Chem Int Ed},
publisher = {Wiley},
abstract = {<jats:p>Electrochemical synthesis of H2O2 by two‐electron oxygen reduction (2e− ORR) often shows limited stability at high current densities in acidic media. Mn‐based catalysts have been demonstrated highly stable for four‐electron ORR thanks to their intrinsically low rate constant for Fenton‐like reactions. However, their activity toward acidic 2e− ORR remains low because of too strong adsorption to *OOH. Here, we report a diatomic Mn catalyst with high‐spin MnII centers to enable high onset potential (0.69 V), high selectivity (> 90%) and outstanding stability (240 h under 300 mA cm−2) towards H2O2 electrosynthesis in acid. Theoretical calculations and in situ spectroscopies reveal that the diatomic Mn sites have downshifted d‐band center and thus weakened adsorption strength for *OOH. Moreover, the inertia of the MnII sites toward the troublesome Fenton‐like reactions leads to the long‐term stability at high current densities. We further demonstrate the functionalization of waste polyethylene (PE) using the high‐concentration H2O2 as produced, which provides a sustainable route toward on‐site upcycling of plastic waste.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Yue Pan, Xiaozhi Liu, Shiyu Zhen, Jianxiong Zhao, Yuhan Wang, Mengshu Ge, Jinrui Zhang, Ying Pan, Lin Gu, Liang Zhang, Dan Zhou, Dong Su, "Alloying Effects on Iron Oxide Redox Pathways: Insights into Sustainable Hydrogen-Based Reduction", J. Phys. Chem. Lett., 16, 22, 2025, 5506-5514.
@article{Pan2025,
title = {Alloying Effects on Iron Oxide Redox Pathways: Insights into Sustainable Hydrogen-Based Reduction},
author = {Yue Pan and Xiaozhi Liu and Shiyu Zhen and Jianxiong Zhao and Yuhan Wang and Mengshu Ge and Jinrui Zhang and Ying Pan and Lin Gu and Liang Zhang and Dan Zhou and Dong Su},
doi = {10.1021/acs.jpclett.5c00954},
issn = {1948-7185},
year = {2025},
date = {2025-06-05},
urldate = {2025-06-05},
journal = {J. Phys. Chem. Lett.},
volume = {16},
number = {22},
pages = {5506--5514},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Yan Zhang, Fuhua Li, Shuwei Li, Zedong Zhang, Zhiyi Sun, Yuhai Dou, Rui Su, Yahe Wu, Liang Zhang, Wenxing Chen, Dingsheng Wang, Yadong Li, "Asymmetric Dual-Atomic Catalyst with Axial Chloride Coordination for Efficient Oxygen Reduction Reaction", Advanced Materials, 2025, 2507478.
@article{https://doi.org/10.1002/adma.202507478,
title = {Asymmetric Dual-Atomic Catalyst with Axial Chloride Coordination for Efficient Oxygen Reduction Reaction},
author = {Yan Zhang and Fuhua Li and Shuwei Li and Zedong Zhang and Zhiyi Sun and Yuhai Dou and Rui Su and Yahe Wu and Liang Zhang and Wenxing Chen and Dingsheng Wang and Yadong Li},
url = {https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adma.202507478},
doi = {https://doi.org/10.1002/adma.202507478},
year = {2025},
date = {2025-05-29},
urldate = {2025-05-29},
journal = {Advanced Materials},
volume = {n/a},
number = {n/a},
pages = {2507478},
abstract = {Abstract Low-platinum-group metal (low-PGM) catalysts play a crucial role in reducing the cost of proton exchange membrane fuel cells (PEMFCs). Dual-atomic catalysts offer valuable solutions due to their exceptional performance. This work explores the application of axial Cl-coordinated Pt─Co dual atoms on N-doped graphitic carbon (Pt1Co1/NC─Cl) catalysts utilizing PtCo dual-atomic catalysts, demonstrating their ability to significantly enhance the acidic oxygen reduction reaction (ORR) catalytic performance of conventional PtCo catalysts. The half-wave potential (E1/2) reaches 0.841 V in a 0.1 M HClO4 solution, and only a reduction of 12 mV in E1/2 is observed after 5000 cycles. Axial Cl proves to be resistant to removal during the electroreduction reaction. Consequently, the use of heteroatom-modulated asymmetric structures can greatly improve the performance of Pt-based catalysts. Incorporating nonmetallic synergistic Pt-group metals presents a promising solution for achieving high-performance Low-PGMs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

An-Zhen Li, Xiongbo Wang, Shuwei Li, Bo-Jun Yuan, Xi Wang, Ruo-Pu Li, Liang Zhang, Bi-Jie Li, Haohong Duan, "Direct Electrooxidation of Ethylene to Ethylene Glycol over 90% Faradaic Efficiency Enabled by Cl– Modification of the Pd Surface", J. Am. Chem. Soc., 147, 12, 2025, 10493-10503.
@article{Li2025,
title = {Direct Electrooxidation of Ethylene to Ethylene Glycol over 90% Faradaic Efficiency Enabled by Cl^{–} Modification of the Pd Surface},
author = {An-Zhen Li and Xiongbo Wang and Shuwei Li and Bo-Jun Yuan and Xi Wang and Ruo-Pu Li and Liang Zhang and Bi-Jie Li and Haohong Duan},
doi = {10.1021/jacs.4c18345},
issn = {1520-5126},
year = {2025},
date = {2025-03-26},
urldate = {2025-03-26},
journal = {J. Am. Chem. Soc.},
volume = {147},
number = {12},
pages = {10493--10503},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiangfu Niu, Shuwei Li, Zheyu Zhang, Haohong Duan, Rui Zhang, Jianqiu Li, Liang Zhang, "Accelerated Optimization of Compositions and Chemical Ordering for Bimetallic Alloy Catalysts Using Bayesian Learning", ACS Catalysis, 2025, 4374-4383.
@article{Niu2025,
title = {Accelerated Optimization of Compositions and Chemical Ordering for Bimetallic Alloy Catalysts Using Bayesian Learning},
author = {Xiangfu Niu and Shuwei Li and Zheyu Zhang and Haohong Duan and Rui Zhang and Jianqiu Li and Liang Zhang},
url = {https://doi.org/10.1021/acscatal.5c00467},
doi = {10.1021/acscatal.5c00467},
year = {2025},
date = {2025-02-26},
urldate = {2025-02-26},
journal = {ACS Catalysis},
pages = {4374-4383},
publisher = {American Chemical Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Ze-Cheng Yao, Jing Chai, Tang Tang, Liang Ding, Zhe Jiang, Jiaju Fu, Xiaoxia Chang, Bingjun Xu, Liang Zhang, Jin-Song Hu, Li-Jun Wan, "Manipulating hydrogenation pathways enables economically viable electrocatalytic aldehyde-to-alcohol valorization", Proc. Natl. Acad. Sci. U.S.A., 122, 8, 2025.
@article{Yao2025,
title = {Manipulating hydrogenation pathways enables economically viable electrocatalytic aldehyde-to-alcohol valorization},
author = {Ze-Cheng Yao and Jing Chai and Tang Tang and Liang Ding and Zhe Jiang and Jiaju Fu and Xiaoxia Chang and Bingjun Xu and Liang Zhang and Jin-Song Hu and Li-Jun Wan},
doi = {10.1073/pnas.2423542122},
issn = {1091-6490},
year = {2025},
date = {2025-02-25},
urldate = {2025-02-25},
journal = {Proc. Natl. Acad. Sci. U.S.A.},
volume = {122},
number = {8},
publisher = {Proceedings of the National Academy of Sciences},
abstract = {<jats:p>
Electrocatalytic reduction (ECR) of furfural represents a sustainable route for biomass valorization. Unfortunately, traditional Cu-catalyzed ECR suffers from diversified product distribution and industrial-incompatible production rates, mainly caused by the intricate mechanism−performance relationship. Here, we manipulate hydrogenation pathways on Cu by introducing ceria as an auxiliary component, which enables the mechanism switching from proton-coupled electron transfer to electrochemical hydrogen-atom transfer (HAT) and thus high-speed furfural-to-furfuryl alcohol electroconversion. Theoretical and kinetic analyses show that oxygen-vacancy-rich ceria delivers an efficient formation−diffusion−hydrogenation chain of H* by diminishing H* adsorption. Spectroscopic characterizations indicate that Cu/ceria interfacial perimeter enriches the local furfural, synergistically lowering the barrier of the rate-determining HAT step across the perimeter. Our Cu/ceria catalyst realizes high-rate HAT-dominated ECR for electrosynthesis of single-product furfuryl alcohol, achieving a high production rate of 19.1 ± 0.4 mol h
<jats:sup>−1</jats:sup>
m
<jats:sup>−2</jats:sup>
and a Faradaic efficiency of 97 ± 1% at an economically viable partial current density of over 0.1 A cm
<jats:sup>−2</jats:sup>
. Our results demonstrate a highly efficient route for biofeedstock valorization with enhanced techno-economic feasibility.
</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Electrocatalytic reduction (ECR) of furfural represents a sustainable route for biomass valorization. Unfortunately, traditional Cu-catalyzed ECR suffers from diversified product distribution and industrial-incompatible production rates, mainly caused by the intricate mechanism−performance relationship. Here, we manipulate hydrogenation pathways on Cu by introducing ceria as an auxiliary component, which enables the mechanism switching from proton-coupled electron transfer to electrochemical hydrogen-atom transfer (HAT) and thus high-speed furfural-to-furfuryl alcohol electroconversion. Theoretical and kinetic analyses show that oxygen-vacancy-rich ceria delivers an efficient formation−diffusion−hydrogenation chain of H* by diminishing H* adsorption. Spectroscopic characterizations indicate that Cu/ceria interfacial perimeter enriches the local furfural, synergistically lowering the barrier of the rate-determining HAT step across the perimeter. Our Cu/ceria catalyst realizes high-rate HAT-dominated ECR for electrosynthesis of single-product furfuryl alcohol, achieving a high production rate of 19.1 ± 0.4 mol h
<jats:sup>−1</jats:sup>
m
<jats:sup>−2</jats:sup>
and a Faradaic efficiency of 97 ± 1% at an economically viable partial current density of over 0.1 A cm
<jats:sup>−2</jats:sup>
. Our results demonstrate a highly efficient route for biofeedstock valorization with enhanced techno-economic feasibility.
</jats:p>

Xiaochen Wang, Ning Zhang, Huishan Shang, Haojie Duan, Zhiyi Sun, Lili Zhang, Yuanting Lei, Xuan Luo, Liang Zhang, Bing Zhang, Wenxing Chen, "Precisely designing asymmetrical selenium-based dual-atom sites for efficient oxygen reduction", Nature Communications, 16, 1, 2025, 470.
@article{Wang2025,
title = {Precisely designing asymmetrical selenium-based dual-atom sites for efficient oxygen reduction},
author = {Xiaochen Wang and Ning Zhang and Huishan Shang and Haojie Duan and Zhiyi Sun and Lili Zhang and Yuanting Lei and Xuan Luo and Liang Zhang and Bing Zhang and Wenxing Chen},
url = {https://doi.org/10.1038/s41467-025-55862-6},
doi = {10.1038/s41467-025-55862-6},
issn = {2041-1723},
year = {2025},
date = {2025-01-07},
urldate = {2025-01-07},
journal = {Nature Communications},
volume = {16},
number = {1},
pages = {470},
abstract = {Owing to their synergistic interactions, dual-atom catalysts (DACs) with well-defined active sites are attracting increasing attention. However, more experimental research and theoretical investigations are needed to further construct explicit dual-atom sites and understand the synergy that facilitates multistep catalytic reactions. Herein, we precisely design a series of asymmetric selenium-based dual-atom catalysts that comprise heteronuclear SeN2–MN2 (Mþinspace=þinspaceFe, Mn, Co, Ni, Cu, Mo, etc.) active sites for the efficient oxygen reduction reaction (ORR). Spectroscopic characterisation and theoretical calculations revealed that heteronuclear selenium atoms can efficiently polarise the charge distribution of other metal atoms through short-range regulation. In addition, compared with the Se or Fe single-atom sites, the SeFe dual-atom sites facilitate a reduction in the conversion energy barrier from *O to *OH via the coadsorption of *O intermediates. Among these designed selenium-based dual-atom catalysts, selenium-iron dual-atom catalysts achieves superior alkaline ORR performance, with a half-wave potential of 0.926þinspaceV vs. a reversible hydrogen electrode. In addition, the SeN2–FeN2-based Zn–air battery has a high specific capacity (764.8þinspacemAhþinspaceg−1) and a maximum power density (287.2þinspacemWþinspacecm−2). This work may provide a good perspective for designing heteronuclear DACs to improve ORR efficiency.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Zhe He, Kailang Li, Tianxiang Chen, Yunchao Feng, Eduardo Villalobos-Portillo, Carlo Marini, Tsz Woon Benedict Lo, Fuyuan Yang, Liang Zhang, Lichen Liu, "High-purity hydrogen production from dehydrogenation of methylcyclohexane catalyzed by zeolite-encapsulated subnanometer platinum-iron clusters", Nature Communications, 16, 1, 2025, 92.
@article{He2025,
title = {High-purity hydrogen production from dehydrogenation of methylcyclohexane catalyzed by zeolite-encapsulated subnanometer platinum-iron clusters},
author = {Zhe He and Kailang Li and Tianxiang Chen and Yunchao Feng and Eduardo Villalobos-Portillo and Carlo Marini and Tsz Woon Benedict Lo and Fuyuan Yang and Liang Zhang and Lichen Liu},
url = {https://doi.org/10.1038/s41467-024-55370-z},
doi = {10.1038/s41467-024-55370-z},
issn = {2041-1723},
year = {2025},
date = {2025-01-02},
urldate = {2025-01-02},
journal = {Nature Communications},
volume = {16},
number = {1},
pages = {92},
abstract = {Liquid organic hydrogen carriers (LOHCs) are considered promising carriers for large-scale H2 storage and transportation, among which the toluene-methylcyclohexane cycle has attracted great attention from industry and academia because of the low cost and its compatibility with the current infrastructure facility for the transportation of chemicals. The large-scale deployment of the H2 storage/transportation plants based on the toluene-methylcyclohexane cycle relies on the use of high-performance catalysts, especially for the H2 release process through the dehydrogenation of methylcyclohexane. In this work, we have developed a highly efficient catalyst for MCH dehydrogenation reaction by incorporating subnanometer PtFe clusters with precisely controlled composition and location within a rigid zeolite matrix. The resultant zeolite-encapsulated PtFe clusters exhibit the up-to-date highest reaction rate for dehydrogenation of methylcyclohexane to toluene, very high chemoselectivity to toluene (enabling the production of H2 with purity >99.9%), remarkably high stability (>2000þinspaceh) and regenerability over consecutive reaction-regeneration cycles.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Cunyuan Gao, Shiyu Zhen, Yutong Wang, Lingwei Wang, Yang Cao, Jinhua Zhan, Liang Zhang, Bin Cai, "Spin effects in regulating the adsorption characteristics of metal ions", Chem. Sci., 2025.
@article{Gao2025,
title = {Spin effects in regulating the adsorption characteristics of metal ions},
author = {Cunyuan Gao and Shiyu Zhen and Yutong Wang and Lingwei Wang and Yang Cao and Jinhua Zhan and Liang Zhang and Bin Cai},
doi = {10.1039/d4sc06477a},
issn = {2041-6539},
year = {2025},
date = {2025-00-00},
urldate = {2025-00-00},
journal = {Chem. Sci.},
publisher = {Royal Society of Chemistry (RSC)},
abstract = {<jats:p>Spin-state modulation in ZnCo<jats:sub>2</jats:sub>O<jats:sub>4</jats:sub> spinel oxides through calcination-induced lattice distortion enhances metal ion sensing performance, with a direct correlation between the Co<jats:sup>3+</jats:sup> spin state and electrochemical behavior.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

2024
Mingze Sun, Helai Huang, Xiangfu Niu, Shuyan Gong, Zhengwen Li, Jinjie Fang, Xiang Liu, Yanjun Chen, Haohong Duan, Zhongbin Zhuang, Satoshi Nagao, Yuki Aoki, Liang Zhang, Zhiqiang Niu, "Grain Boundary-Derived Local Amorphization Enhances Acidic OER", ACS Catalysis, 2024, 15764-15776.
@article{Sun2024,
title = {Grain Boundary-Derived Local Amorphization Enhances Acidic OER},
author = {Mingze Sun and Helai Huang and Xiangfu Niu and Shuyan Gong and Zhengwen Li and Jinjie Fang and Xiang Liu and Yanjun Chen and Haohong Duan and Zhongbin Zhuang and Satoshi Nagao and Yuki Aoki and Liang Zhang and Zhiqiang Niu},
url = {https://doi.org/10.1021/acscatal.4c03746},
doi = {10.1021/acscatal.4c03746},
year = {2024},
date = {2024-10-09},
urldate = {2024-10-09},
journal = {ACS Catalysis},
pages = {15764-15776},
publisher = {American Chemical Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Zhiyi Sun, Xuan Luo, Huishan Shang, Ziding Wang, Liang Zhang, Wenxing Chen, "Atomic Printing Strategy Achieves Precise Anchoring of Dual-Copper Atoms on C2N Structure for Efficient CO2 Reduction to Ethylene", Angewandte Chemie International Edition, 2024, e202405778.
@article{Sun2024b,
title = {Atomic Printing Strategy Achieves Precise Anchoring of Dual-Copper Atoms on C2N Structure for Efficient CO2 Reduction to Ethylene},
author = {Zhiyi Sun and Xuan Luo and Huishan Shang and Ziding Wang and Liang Zhang and Wenxing Chen},
url = {https://doi.org/10.1002/anie.202405778},
doi = {10.1002/anie.202405778},
issn = {1433-7851},
year = {2024},
date = {2024-09-09},
urldate = {2024-09-09},
journal = {Angewandte Chemie International Edition},
pages = {e202405778},
publisher = {John Wiley & Sons, Ltd},
abstract = {Isolated metal sites catalysts (IMSCs) play crucial role in electrochemical CO2 reduction, with potential industrial applications. However, tunable synthesis strategies for IMSCs are limited. Herein, we present an atomic printing strategy that draws inspiration from the ancient Chinese 'movable-type printing technology'. Selecting customizable combinations of metal atoms as metal precursors form an extensive binuclear metal library. A series of dual-atom catalysts were prepared by utilizing the edge nitrogen atoms in the C2N cavity as anchoring 'pincers' to capture metal atoms. To prove utility, the dual atom catalyst Cu2-C2N is investigated as electrocatalytic CO2RR catalyst. The synergistic interaction of dual Cu atoms promotes C-C coupling and guarantees FEC2+ (90.8%) and FEC2H4. (71.7%) at -1.10 V vs RHE. DFT calculations revealed the Cu2 site would be subtly flipped during CO2RR for enhancing *CO adsorption and dimerization. We validate that atomic printing strategies are applicable to wide range of metal combinations, representing a significant advancement in the development of IMSCs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiaomeng Dou, Kailang Li, Kun Zhang, Chaofeng Zhu, Debora M. Meira, Yang Song, Peng He, Liang Zhang, Lichen Liu, "Isolated Pt Atoms Stabilized by Ga2O3 Clusters Confined in ZSM-5 for Nonoxidative Activation of Ethane", JACS Au, 2024.
@article{Dou2024,
title = {Isolated Pt Atoms Stabilized by Ga_{2}O_{3} Clusters Confined in ZSM-5 for Nonoxidative Activation of Ethane},
author = {Xiaomeng Dou and Kailang Li and Kun Zhang and Chaofeng Zhu and Debora M. Meira and Yang Song and Peng He and Liang Zhang and Lichen Liu},
doi = {10.1021/jacsau.4c00480},
issn = {2691-3704},
year = {2024},
date = {2024-08-26},
urldate = {2024-08-26},
journal = {JACS Au},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiaoyun Miao, Jiangyuan Feng, Zhongqin Dai, Xingzhi Zhu, Jianjun Wen, Liang Zhang, Xiaofeng Ye, Yucun Zhou, Zhaoyin Wen, "A Regenerative Coking‐resistant CO2 Hydrogenation Reactor using a Protonic Ceramic Electrolysis Cell with Thin and Robust Fuel Electrode", Advanced Energy Materials, 2024.
@article{Miao2024,
title = {A Regenerative Coking‐resistant CO_{2} Hydrogenation Reactor using a Protonic Ceramic Electrolysis Cell with Thin and Robust Fuel Electrode},
author = {Xiaoyun Miao and Jiangyuan Feng and Zhongqin Dai and Xingzhi Zhu and Jianjun Wen and Liang Zhang and Xiaofeng Ye and Yucun Zhou and Zhaoyin Wen},
doi = {10.1002/aenm.202402208},
issn = {1614-6840},
year = {2024},
date = {2024-07-30},
urldate = {2024-07-30},
journal = {Advanced Energy Materials},
publisher = {Wiley},
abstract = {<jats:title>Abstract</jats:title><jats:p>A CO<jats:sub>2</jats:sub> hydrogenation reactor based on protonic ceramic electrolysis cells (PCECs) is one of the most promising options for the efficient and clean conversion of CO<jats:sub>2</jats:sub>. However, insufficient performance and durability have hindered the practical application of such CO<jats:sub>2</jats:sub> hydrogenation reactors. Here, fabricates a large‐area (≈10 cm<jats:sup>2</jats:sup>) tubular air electrode supported PCEC (AES‐PCEC) and develop a fuel electrode regeneration strategy for efficient and durable CO<jats:sub>2</jats:sub> hydrogenation. The AES‐PCEC demonstrates a CO yield of 2.88 mL min<jats:sup>−1</jats:sup> at 650 °C while maintaining excellent durability for over 1100 h. The high stability of the AES‐PCEC can be attributed to the robust and thin fuel electrode structure as well as the water‐mediated carbon removal regeneration mechanism. Density functional theory calculations have confirmed the regeneration mechanism facilitated by the excellent water hydration and dissociation capability of the BaCe<jats:sub>0.4</jats:sub>Zr<jats:sub>0.4</jats:sub>Y<jats:sub>0.1</jats:sub>Yb<jats:sub>0.1</jats:sub>O<jats:sub>3‐σ.</jats:sub> This work offers a feasible strategy to design high‐performance and durable PCEC‐based CO<jats:sub>2</jats:sub> hydrogenation reactors.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiaochen Wang, Ning Zhang, Shuohai Guo, Huishan Shang, Xuan Luo, Zhiyi Sun, Zihao Wei, Yuanting Lei, Lili Zhang, Dan Wang, Yafei Zhao, Fang Zhang, Liang Zhang, Xu Xiang, Wenxing Chen, Bing Zhang, "p-d Orbital Hybridization Induced by Asymmetrical FeSn Dual Atom Sites Promotes the Oxygen Reduction Reaction", J. Am. Chem. Soc., 146, 31, 2024, 21357-21366.
@article{Wang2024,
title = {p-d Orbital Hybridization Induced by Asymmetrical FeSn Dual Atom Sites Promotes the Oxygen Reduction Reaction},
author = {Xiaochen Wang and Ning Zhang and Shuohai Guo and Huishan Shang and Xuan Luo and Zhiyi Sun and Zihao Wei and Yuanting Lei and Lili Zhang and Dan Wang and Yafei Zhao and Fang Zhang and Liang Zhang and Xu Xiang and Wenxing Chen and Bing Zhang},
doi = {10.1021/jacs.4c03576},
issn = {1520-5126},
year = {2024},
date = {2024-07-25},
urldate = {2024-07-25},
journal = {J. Am. Chem. Soc.},
volume = {146},
number = {31},
pages = {21357--21366},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Shuwei Li, Liang Zhang, "Accurate first-principles simulation for the response of 2D chemiresistive gas sensors", npj Comput Mater, 10, 1, 2024.
@article{Li2024,
title = {Accurate first-principles simulation for the response of 2D chemiresistive gas sensors},
author = {Shuwei Li and Liang Zhang},
doi = {10.1038/s41524-024-01329-z},
issn = {2057-3960},
year = {2024},
date = {2024-06-29},
urldate = {2024-06-29},
journal = {npj Comput Mater},
volume = {10},
number = {1},
publisher = {Springer Science and Business Media LLC},
abstract = {<jats:title>Abstract</jats:title><jats:p>The realm of chemiresistive gas sensors has witnessed a notable surge in interest in two-dimensional (2D) materials. The advancement of high-performance 2D gas sensing materials necessitates a quantitative theoretical method capable of accurately predicting their response. In this context, we present our first-principles framework for calculating the response of 2D materials, incorporating both carrier concentration and mobility. We showcase our method by applying it to prototype NH<jats:sub>3</jats:sub> sensing on 2D MoS<jats:sub>2</jats:sub> and comparing the results with prior experiments in the literature. Our approach offers a thorough solution for carrier concentration, taking into account the electronic structure around the Fermi level. In conjunction with the mobility calculation, this enables us to provide a quantitative prediction of the response profile and limit of detection (LOD), yielding a notably improved alignment with prior experimental findings. Further analysis quantifies the contributions of carrier concentration and mobility to the overall response of 2D MoS<jats:sub>2</jats:sub> to NH<jats:sub>3</jats:sub>. We identify that discrepancies in the charge-transfer-based method primarily stem from overestimating carrier concentrations. Our method opens exciting opportunities to explore carrier mobility-dominated sensing materials, facilitates efficient screening of promising gas sensing materials, and quantitative understanding of the sensing mechanism.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiaozhi Liu, Yue Pan, Jianxiong Zhao, Yuhan Wang, Mengshu Ge, Lixiang Qian, Liang Zhang, Lin Gu, Dan Zhou, Dong Su, "Atomically Resolved Transition Pathways of Iron Redox", J. Am. Chem. Soc., 146, 25, 2024, 17487-17494.
@article{Liu2024,
title = {Atomically Resolved Transition Pathways of Iron Redox},
author = {Xiaozhi Liu and Yue Pan and Jianxiong Zhao and Yuhan Wang and Mengshu Ge and Lixiang Qian and Liang Zhang and Lin Gu and Dan Zhou and Dong Su},
doi = {10.1021/jacs.4c05309},
issn = {1520-5126},
year = {2024},
date = {2024-06-12},
urldate = {2024-06-12},
journal = {J. Am. Chem. Soc.},
volume = {146},
number = {25},
pages = {17487--17494},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Li, Yaqiong, Luo, Xuan, Zhang, Fang, Sun, Zhiyi, Wei, Zihao, Deng, Ziwei, Zhan, Ziheng, Zhao, Chaofeng, Sun, Qi, Zhang, Liang, Chen, Wenxing, Li, Sheng-Hua, Pang, Si-Ping, "Precisely Constructing Charge-Asymmetric Dual-Atom Fe Sites Supported on Hollow Porous Carbon Spheres for Efficient Oxygen Reduction", Energy & Environmental Science, 17, 13, 2024, 4646-4657.
@article{D4EE01309C,
title = {Precisely Constructing Charge-Asymmetric Dual-Atom Fe Sites Supported on Hollow Porous Carbon Spheres for Efficient Oxygen Reduction},
author = {Li, Yaqiong and Luo, Xuan and Zhang, Fang and Sun, Zhiyi and Wei, Zihao and Deng, Ziwei and Zhan, Ziheng and Zhao, Chaofeng and Sun, Qi and Zhang, Liang and Chen, Wenxing and Li, Sheng-Hua and Pang, Si-Ping},
doi = {10.1039/D4EE01309C},
issn = {1754-5692},
year = {2024},
date = {2024-05-30},
urldate = {2024-05-30},
journal = {Energy & Environmental Science},
volume = {17},
issue = {13},
pages = {4646-4657},
abstract = {The transition group metal catalysts showing atomic dispersion are on the rise as affordable electrocatalysts for oxygen reduction reaction (ORR) in fuel cell batteries, but their activity in acidic media remains constrained. In this work, we present a new catalyst (Fe2-S1N5/SNC) featuring for the ORR. The active site of catalysis composed of two charge-asymmetric iron atoms can regulate the adsorption energy of intermediate species (OH*) in the ORR reaction, thereby enhancing the kinetics of the ORR reaction. Furthermore, the use of hollow porous carbon spheres as the support for the catalysis enhances substance transport during the ORR reaction. The Fe2-S1N5/SNC exhibits remarkable electrochemical activity in ORR, displaying a remarkably high half-wave potential of 0.829 V vs. RHE, and it also demonstrates impressive stability, as the half-wave potential only decreases by 26 mV after 5000 cycles in a 0.1 M HClO4 solution. Therefore, this study offers valuable insights into the low-cost metal catalysts design for ORR, specifically the precise construction of active sites.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiaomeng Dou, Tao Yan, Lixiang Qian, Huaming Hou, Miguel Lopez-Haro, Carlo Marini, Giovanni Agostini, Debora M Meira, Xiangjie Zhang, Liang Zhang, Zhi Cao, Lichen Liu, "Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite", Nature Catalysis, 7, 6, 2024, 666-677.
@article{Dou1929b,
title = {Regioselective hydroformylation with subnanometre Rh clusters in MFI zeolite},
author = {Xiaomeng Dou and Tao Yan and Lixiang Qian and Huaming Hou and Miguel Lopez-Haro and Carlo Marini and Giovanni Agostini and Debora M Meira and Xiangjie Zhang and Liang Zhang and Zhi Cao and Lichen Liu},
url = {https://zhanglab-thu.com/wp-content/uploads/2024/05/s41929-024-01155-y-3.pdf},
doi = {10.1038/s41929-024-01155-y},
issn = {2520-1158},
year = {2024},
date = {2024-05-15},
urldate = {2024-05-15},
journal = {Nature Catalysis},
volume = {7},
issue = {6},
pages = {666-677},
abstract = {Achieving the regioselective hydroformylation of linear α-olefins to linear aldehydes using solid catalysts with regioselectivities comparable to the corresponding homogeneous process is a great challenge in the chemical industry. Despite the tremendous efforts devoted to this research topic, most of the reported heterogeneous metal catalysts still give considerably lower regioselectivities than well-established homogeneous metal catalysts. Here we show the design of efficient Rh-zeolite catalysts, in which subnanometre Rh clusters are selectively confined in the sinusoidal ten-membered-ring channels of MFI zeolite, for the hydroformylation of long-chain linear α-olefins (C 6-C 12) into linear aldehydes with very high linear-to-branched aldehyde ratios (up to 400). The exceptional catalytic performances result from the involvement of the MFI zeolite framework as a rigid solid ligand that accommodates subnanometre Rh clusters in the sinusoidal channels of the MFI zeolite. Transition-metal-catalysed hydroformylation represents a premier route for the conversion of olefins into broadly applicable aldehydes and constitutes one of the largest homogeneous catalytic processes in the chemical industry (Fig. 1a) 1-3. Tremendous efforts have been devoted to the development of catalysts with high linear-to-branched (l/b) ratios of aldehydes because linear aldehydes and the derived alcohols are more desirable than the branched isomers in downstream processes (Fig. 1b) 4-7. Issues of unavoidable catalyst degradation , the necessary use of excessive/expensive ligands and catalyst separation/recycling impede further upgrading of the process and limit the adoption of olefins from different feedstock streams. In comparison with their homogeneous counterparts, heterogeneous catalysts are advantageous in terms of their separation/ recycling and their feasibility for implementation in continuous processes. Enduring efforts have been made to develop heterogeneous hydroformylation catalysts through multiple approaches (Fig. 1c) 8-15. However, none of these strategies can meet the high regioselectivity of the related homogeneous catalysts 16-19. To overcome the abovementioned limitations, we sought to investigate subnanometre Rh clusters confined in zeolites as tunable catalysts for olefin hydroformylation reactions, as the crystalline micropo-rous channels/cavities can not only accommodate the Rh sites with high structural uniformity but also provide a well-defined coordination environment for the Rh sites to constrain the transition states of the hydroformylation reaction for the selective production of linear aldehydes.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xiaoyu Chen, Jiawei Wan, Jing Chai, Liang Zhang, Fang Zhang, Qinghua Zhang, Lin Gu, Lirong Zheng, Ranbo Yu, "Nickel-iron in the second coordination shell boost single-atomic-site iridium catalysts for high-performance urea electrooxidation", Nano Res., 17, 5, 2024, 3919-3926.
@article{Chen2024,
title = {Nickel-iron in the second coordination shell boost single-atomic-site iridium catalysts for high-performance urea electrooxidation},
author = {Xiaoyu Chen and Jiawei Wan and Jing Chai and Liang Zhang and Fang Zhang and Qinghua Zhang and Lin Gu and Lirong Zheng and Ranbo Yu},
doi = {10.1007/s12274-023-6388-1},
issn = {1998-0000},
year = {2024},
date = {2024-05-00},
urldate = {2024-05-00},
journal = {Nano Res.},
volume = {17},
number = {5},
pages = {3919--3926},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
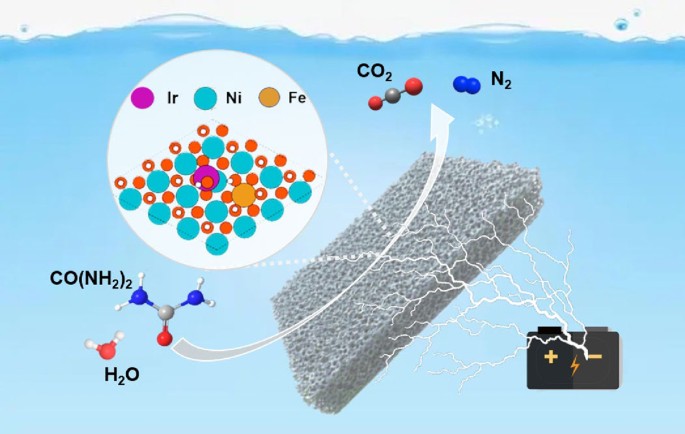
Yuhan Wang, Xincheng Lei, Jianxiong Zhao, Xiaozhi Liu, Liang Zhang, Dong Su, "Structural engineering of Pt-based intermetallic catalysts", Journal of Materials Research, 39, 9, 2024, 1325-1343.
@article{Wang2024b,
title = {Structural engineering of Pt-based intermetallic catalysts},
author = {Yuhan Wang and Xincheng Lei and Jianxiong Zhao and Xiaozhi Liu and Liang Zhang and Dong Su},
doi = {10.1557/s43578-024-01329-1},
issn = {2044-5326},
year = {2024},
date = {2024-04-02},
urldate = {2024-04-02},
journal = {Journal of Materials Research},
volume = {39},
number = {9},
pages = {1325--1343},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

RuYang Shao, Xiangfu Niu, XiaoChu Xu, Zhen-Hua Zhou, Shengqi Chu, Lei Tong, Liang Zhang, HaiWei Liang, "Enhancing Surface Strain of Intermetallic Fuel Cell Catalysts by Composition-Induced Phase Transition", Nano Lett, 24, 18, 2024, 5578–5584.
@article{pmid38682925,
title = {Enhancing Surface Strain of Intermetallic Fuel Cell Catalysts by Composition-Induced Phase Transition},
author = {RuYang Shao and Xiangfu Niu and XiaoChu Xu and Zhen-Hua Zhou and Shengqi Chu and Lei Tong and Liang Zhang and HaiWei Liang},
doi = {10.1021/acs.nanolett.4c00898},
issn = {1530-6992},
year = {2024},
date = {2024-04-01},
urldate = {2024-04-01},
journal = {Nano Lett},
volume = {24},
issue = {18},
pages = { 5578–5584},
abstract = {The lattice parameter of platinum-based intermetallic compounds (IMCs), which correlates with the intrinsic activity of the oxygen reduction reaction (ORR), can be modulated by crystal phase engineering. However, the controlled preparation of IMCs with unconventional crystal structures remains highly challenging. Here, we demonstrate the synthesis of carbon-supported PtCu-based IMC catalysts with an unconventional L1 structure by a composition-regulated strategy. Experiment and machine learning reveal that the thermodynamically favorable structure changes from L1 to L1 when slight Cu atoms are substituted with Co. Benefiting from crystal-phase-induced strain enhancement, the prepared L1-type PtCuCo catalyst exhibits much-enhanced mass and specific activities of 1.82 A mg and 3.27 mA cm, which are 1.91 and 1.73 times higher than those of the L1-type PtCu catalyst, respectively. Our work highlights the important role of crystal phase in determining the surface strain of IMCs, and opens a promising avenue for the rational preparation of IMCs with different crystal phases by doping.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Huang, Helai, Sun, Mingze, Li, Shuwei, Zhang, Shengbo, Lee, Yiyang, Li, Zhengwen, Fang, Jinjie, Chen, Chengjin, Zhang, Yu-Xiao, Wu, Yanfen, Che, Yizhen, Qian, Shuairen, Zhu, Wei, Tang, Cheng, Zhuang, Zhongbin, Zhang, Liang, Niu, Zhiqiang, "Enhancing H2O2 Electrosynthesis at Industrial-Relevant Current in Acidic Media on Diatomic Cobalt Sites", Journal of the American Chemical Society, 146, 13, 2024, 9434-9443.
@article{nokey,
title = {Enhancing H2O2 Electrosynthesis at Industrial-Relevant Current in Acidic Media on Diatomic Cobalt Sites},
author = {Huang, Helai and Sun, Mingze and Li, Shuwei and Zhang, Shengbo and Lee, Yiyang and Li, Zhengwen and Fang, Jinjie and Chen, Chengjin and Zhang, Yu-Xiao and Wu, Yanfen and Che, Yizhen and Qian, Shuairen and Zhu, Wei and Tang, Cheng and Zhuang, Zhongbin and Zhang, Liang and Niu, Zhiqiang},
doi = {10.1021/jacs.4c02031},
issn = {0002-7863},
year = {2024},
date = {2024-03-20},
journal = {Journal of the American Chemical Society},
volume = {146},
issue = {13},
pages = {9434-9443},
abstract = {Electrocatalytic synthesis of hydrogen peroxide (H2O2) in acidic media is an efficient and eco-friendly approach to produce inherently stable H2O2, but limited by the lack of selective and stable catalysts under industrial-relevant current densities. Herein, we report a diatomic cobalt catalyst for two-electron oxygen reduction to efficiently produce H2O2 at 50–400 mA cm–2 in acid. Electrode kinetics study shows a >95% selectivity for two-electron oxygen reduction on the diatomic cobalt sites. In a flow cell device, a record-high production rate of 11.72 mol gcat–1 h–1 and exceptional long-term stability (100 h) are realized under high current densities. In situ spectroscopic studies and theoretical calculations reveal that introducing a second metal into the coordination sphere of the cobalt site can optimize the binding strength of key H2O2 intermediates due to the downshifted d-band center of cobalt. We also demonstrate the feasibility of processing municipal plastic wastes through decentralized H2O2 production.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Changli Chen, Jing Chai, Mengru Sun, Tianqi Guo, Jie Lin, Yurong Zhou, Zhiyi Sun, Fang Zhang, Liang Zhang, Wenxing Chen, Yujing Li, "An asymmetrically coordinated ZnCoFe hetero-trimetallic atom catalyst enhances the electrocatalytic oxygen reaction", Energy & Environmental Science, 17, 6, 2024, 2298-2308.
@article{D4EE00134F,
title = {An asymmetrically coordinated ZnCoFe hetero-trimetallic atom catalyst enhances the electrocatalytic oxygen reaction},
author = {Changli Chen and Jing Chai and Mengru Sun and Tianqi Guo and Jie Lin and Yurong Zhou and Zhiyi Sun and Fang Zhang and Liang Zhang and Wenxing Chen and Yujing Li},
url = {http://dx.doi.org/10.1039/D4EE00134F},
doi = {10.1039/D4EE00134F},
issn = {1754-5692},
year = {2024},
date = {2024-02-15},
urldate = {2024-02-15},
journal = {Energy & Environmental Science},
volume = {17},
issue = {6},
pages = {2298-2308},
publisher = {The Royal Society of Chemistry},
abstract = {Synthesizing heterometal atomic sites with asymmetric coordination structures is of great significance for improving the electrocatalytic performance of atomically dispersed catalysts, yet it is also a challenge. Herein, an unusual ZnCoFe hetero-trimetallic atom site is elaborately developed with the nitrogen-coordinated Co and Zn atoms adjacent to the sulfur/nitrogen dual-coordinated Fe atoms (ZnN3CoN3FeN2S) anchored in sulfur/nitrogen-doped carbon via a simple two-step wet chemistry strategy based on metal-organic framework (MOF) and post-coordination process. The ZnCoFe-TAC/SNC shows the smallest ∆E of 0.676 V, indicating an outstanding bifunctional catalytic activity. Further, the ZnCoFe-TAC/SNC-based Zn-air battery displays high peak power density (304 mW cm-2) and specific capacity (760 mAh g-1). The in-situ XAS results show that Co is the main active site, and Fe is a co-catalytic site. Zn acts as an “electron regulator” to regulate the electron structures around the catalytic sites. Density functional theory (DFT) calculations further reveal the synergetic effect of the interactions among Zn, Co, and Fe metal atoms on the catalytic performance. This work provides a universal insight into the controllable synthesis of trimetallic atom catalysts and a proposal for regulating the performance in energy conversion and catalytic applications.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Rui Sui, Jing Chai, Xuerui Liu, Jiajing Pei, Xuejiang Zhang, Xingdong Wang, Yu Wang, Juncai Dong, Wei Zhu, Wenxing Chen, Liang Zhang, Zhongbin Zhuang, "Introducing highly polarizable cation in M-N-C type catalysts to boost their oxygen reduction reaction performance", Applied Catalysis B: Environmental, 341, 2024, 123251.
@article{SUI2024123251,
title = {Introducing highly polarizable cation in M-N-C type catalysts to boost their oxygen reduction reaction performance},
author = {Rui Sui and Jing Chai and Xuerui Liu and Jiajing Pei and Xuejiang Zhang and Xingdong Wang and Yu Wang and Juncai Dong and Wei Zhu and Wenxing Chen and Liang Zhang and Zhongbin Zhuang},
url = {https://www.sciencedirect.com/science/article/pii/S0926337323008949},
doi = {https://doi.org/10.1016/j.apcatb.2023.123251},
issn = {0926-3373},
year = {2024},
date = {2024-02-01},
urldate = {2024-02-01},
journal = {Applied Catalysis B: Environmental},
volume = {341},
pages = {123251},
abstract = {The metal-nitrogen-carbon (M-N-C) type oxygen reduction reaction (ORR) catalysts are promising for hydroxide exchange membrane fuel cells, but catalysts with further improved performance are challenging. Here, we report the efficient improvement of the ORR activity of M-N-C catalysts by employing highly polarizable metal cation Ag+. Ag, Fe single atomic sites embedded in concave nitrogen doped carbon (Ag1Fe1/CNC) is successfully synthesized and shows high performance towards ORR, indicating by both the ultra-high half-wave potential of 0.917 V and membrane electrolyte assembly performance of peak power density up to 1.26 W cm−2. The binding energy of the ORR intermediates on the highly polarizable Ag site in Ag1Fe1/CNC was significantly tuned by the adjacent Fe site by more than 0.2 eV, and thus leading to a low theoretical ORR overpotential of only 0.398 V. The employment of the highly polarizable metal cation brings a novel and efficient approach to construct highly active catalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Peng Yin, Xiangfu Niu, Shuo-bin Li, Kai Chen, Xi Zhang, Ming Zuo, Liang Zhang, Hai-Wei Liang, "Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts", Nature Communications, 15, 1, 2024, 415.
@article{nokey,
title = {Machine-learning-accelerated design of high-performance platinum intermetallic nanoparticle fuel cell catalysts},
author = {Peng Yin and Xiangfu Niu and Shuo-bin Li and Kai Chen and Xi Zhang and Ming Zuo and Liang Zhang and Hai-Wei Liang},
url = {https://doi.org/10.1038/s41467-023-44674-1},
doi = {10.1038/s41467-023-44674-1},
issn = {2041-1723},
year = {2024},
date = {2024-01-10},
urldate = {2024-01-10},
journal = {Nature Communications},
volume = {15},
issue = {1},
pages = {415},
abstract = {Carbon supported PtCo intermetallic alloys are known to be one of the most promising candidates as low-platinum oxygen reduction reaction electrocatalysts for proton-exchange-membrane fuel cells. Nevertheless, the intrinsic trade-off between particle size and ordering degree of PtCo makes it challenging to simultaneously achieve a high specific activity and a large active surface area. Here, by machine-learning-accelerated screenings from the immense configuration space, we are able to statistically quantify the impact of chemical ordering on thermodynamic stability. We find that introducing of Cu/Ni into PtCo can provide additional stabilization energy by inducing Co-Cu/Ni disorder, thus facilitating the ordering process and achieveing an improved tradeoff between specific activity and active surface area. Guided by the theoretical prediction, the small sized and highly ordered ternary Pt2CoCu and Pt2CoNi catalysts are experimentally prepared, showing a large electrochemically active surface area of ~90 m2 gPt‒1 and a high specific activity of ~3.5 mA cm‒2.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

2023
Hongfei Gu, Jiani Li, Xiangfu Niu, Jie Lin, Li-Wei Chen, Zedong Zhang, Ziqian Shi, Zhiyi Sun, Qingqing Liu, Peng Zhang, Wensheng Yan, Yu Wang, Liang Zhang, Pengfei Li, Xinyuan Li, Dingsheng Wang, Penggang Yin, Wenxing Chen, "Symmetry-Breaking -Block Antimony Single Atoms Trigger N-Bridged Titanium Sites for Electrocatalytic Nitrogen Reduction with High Efficiency", ACS Nano, 2023.
@article{pmid37909679,
title = {Symmetry-Breaking -Block Antimony Single Atoms Trigger N-Bridged Titanium Sites for Electrocatalytic Nitrogen Reduction with High Efficiency},
author = {Hongfei Gu and Jiani Li and Xiangfu Niu and Jie Lin and Li-Wei Chen and Zedong Zhang and Ziqian Shi and Zhiyi Sun and Qingqing Liu and Peng Zhang and Wensheng Yan and Yu Wang and Liang Zhang and Pengfei Li and Xinyuan Li and Dingsheng Wang and Penggang Yin and Wenxing Chen},
doi = {10.1021/acsnano.3c07857},
issn = {1936-086X},
year = {2023},
date = {2023-11-01},
urldate = {2023-11-01},
journal = {ACS Nano},
abstract = {The electrochemical nitrogen reduction reaction (eNRR) under mild conditions emerges as a promising approach to produce ammonia (NH) compared to the typical Haber-Bosch process. Herein, we design an asymmetrically coordinated -block antimony single-atom catalyst immobilized on nitrogen-doped TiCT (Sb SA/N-TiCT) for eNRR, which exhibits ultrahigh NH yield (108.3 μg h mg) and excellent Faradaic efficiency (41.2%) at -0.3 V vs RHE. Complementary spectroscopies with theoretical calculations reveal that the nitrogen-bridged two titanium atoms triggered by an adjacent asymmetrical Sb-NC moiety act as the active sites for facilitating the protonation of the rate-determining step from *N to *NH and the kinetic conversion of key intermediates during eNRR. Moreover, the introduction of Sb-NC promotes the formation of oxygen vacancies to expose more titanium sites. This work presents a strategy for single-atom-decorated ultrathin two-dimensional materials with the aim of simultaneously enhancing NH yield and Faradaic efficiency for electrocatalytic nitrogen reduction.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Ningyue Chen, Shuwei Li, Peng Zhao, Ran Liu, Yu Xie, Jin-Liang Lin, Christian A. Nijhuis, Bingqian Xu, Liang Zhang, Huaping Xu, Yuan Li, "Extreme Long-Lifetime Self-Assembled Monolayer for Air-Stable Molecular Junctions", Science Advances, 9, 42, 2023, eadh3412.
@article{doi:10.1126/sciadv.adh3412,
title = {Extreme Long-Lifetime Self-Assembled Monolayer for Air-Stable Molecular Junctions},
author = {Ningyue Chen and Shuwei Li and Peng Zhao and Ran Liu and Yu Xie and Jin-Liang Lin and Christian A. Nijhuis and Bingqian Xu and Liang Zhang and Huaping Xu and Yuan Li},
url = {https://doi.org/10.1126/sciadv.adh3412},
doi = {10.1126/sciadv.adh3412},
year = {2023},
date = {2023-08-18},
urldate = {2023-08-18},
journal = {Science Advances},
volume = {9},
issue = {42},
pages = {eadh3412},
abstract = {The molecular electronic devices based on self-assembled monolayer (SAM) on metal surfaces demonstrate novel electronic functions for device minimization yet are unable to realize in practical applications, due to their instability against oxidation of the sulfur-metal bond. This paper describes an alternative to the thiolate anchoring group to form stable SAMs on gold by selenides anchoring group. Because of the formation of strong selenium-gold bonds, these stable SAMs allow us to incorporate them in molecular tunnel junctions to yield extremely stable junctions for over 200 days. A detailed structural characterization supported by spectroscopy and first-principles modeling shows that the oxidation process is much slower with the selenium-gold bond than the sulfur-gold bond, and the selenium-gold bond is strong enough to avoid bond breaking even when it is eventually oxidized. This proof of concept demonstrates that the extraordinarily stable SAMs derived from selenides are useful for long-lived molecular electronic devices and can possibly become important in many air-stable applications involving SAMs. The air stability of molecular junctions is improved for more than 200 days by changing anchoring groups from thiols to selenides.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Wuxian Peng, Ningyue Chen, Caiyun Wang, Yu Xie, Shengzhe Qiu, Shuwei Li, Liang Zhang, Yuan Li, "Fine-Tuning the Molecular Design for High-Performance Molecular Diodes Based on Pyridyl Isomers", Angewandte Chemie International Edition, 62, 34, 2023, e202307733.
@article{https://doi.org/10.1002/anie.202307733,
title = {Fine-Tuning the Molecular Design for High-Performance Molecular Diodes Based on Pyridyl Isomers},
author = {Wuxian Peng and Ningyue Chen and Caiyun Wang and Yu Xie and Shengzhe Qiu and Shuwei Li and Liang Zhang and Yuan Li},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202307733},
doi = {https://doi.org/10.1002/anie.202307733},
issn = {1433-7851},
year = {2023},
date = {2023-07-04},
urldate = {2023-07-04},
journal = {Angewandte Chemie International Edition},
volume = {62},
issue = {34},
pages = {e202307733},
abstract = {Abstract Better control of molecule-electrode coupling (Γ) to minimize leakage current is an effective method to optimize the functionality of molecular diodes. Herein we embedded 5 isomers of phenypyridyl derivatives, each with an N atom placed at a different position, in two electrodes to fine-tune Γ between self-assembled monolayers (SAMs) and the top electrode of EGaIn (eutectic Ga−In terminating in Ga2O3). Combined with electrical tunnelling results, characterizations of electronic structures, single-level model fittings, and DFT calculations, we found that the values of Γ of SAMs formed by these isomers could be regulated by nearly 10 times, thereby contributing to the leakage current changing over about two orders of magnitude and switching the isomers from resistors to diodes with a rectification ratio (r+=|J(+1.5 V)/J(−1.5 V)|) exceeding 200. We demonstrated that the N atom placement can be chemically engineered to tune the resistive and rectifying properties of the molecular junctions, making it possible to convert molecular resistors into rectifiers. Our study provides fundamental insights into the role of isomerism in molecular electronics and offers a new avenue for designing functional molecular devices.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Tang, Tang, Liu, XiaoZhi, Luo, Xuan, Xue, Zhuangzhuang, Pan, Hai-Rui, Fu, Jiaju, Yao, Ze-Cheng, Jiang, Zhe, Lyu, Zhen-Hua, Zheng, Lirong, Su, Dong, Zhang, Jia-Nan, Zhang, Liang, Hu, Jin-Song, "Unconventional Bilateral Compressive Strained Ni–Ir Interface Synergistically Accelerates Alkaline Hydrogen Oxidation", Journal of the American Chemical Society, 145, 25, 2023, 13805–13815.
@article{doi:10.1021/jacs.3c02487,
title = {Unconventional Bilateral Compressive Strained Ni–Ir Interface Synergistically Accelerates Alkaline Hydrogen Oxidation},
author = {Tang, Tang and Liu, XiaoZhi and Luo, Xuan and Xue, Zhuangzhuang and Pan, Hai-Rui and Fu, Jiaju and Yao, Ze-Cheng and Jiang, Zhe and Lyu, Zhen-Hua and Zheng, Lirong and Su, Dong and Zhang, Jia-Nan and Zhang, Liang and Hu, Jin-Song},
url = {https://doi.org/10.1021/jacs.3c02487},
doi = {10.1021/jacs.3c02487},
year = {2023},
date = {2023-06-28},
urldate = {2023-06-28},
journal = {Journal of the American Chemical Society},
volume = {145},
number = {25},
pages = {13805–13815},
abstract = {The alkaline hydrogen oxidation reaction (HOR) involves the coupling of adsorbed hydrogen (Had) and hydroxyl (OHad) species and is thus orders of magnitude slower than that in acid media. According to the Sabatier principle, developing electrocatalysts with appropriate binding energy for both intermediates is vital to accelerating the HOR though it is still challenging. Herein, we propose an unconventional bilateral compressive strained Ni–Ir interface (Ni–Ir(BCS)) as efficient synergistic HOR sites. Density functional theory (DFT) simulations reveal that the bilateral compressive strain effect leads to the appropriate adsorption for both Had and OHad, enabling their coupling thermodynamically spontaneous and kinetically preferential. Such Ni–Ir(BCS) is experimentally achieved by embedding sub-nanometer Ir clusters in graphene-loaded high-density Ni nanocrystals (Ni–Ir(BCS)/G). As predicted, it exhibits a HOR mass activity of 7.95 and 2.88 times those of commercial Ir/C and Pt/C together with much enhanced CO tolerance, respectively, ranking among the most active state-of-the-art HOR catalysts. These results provide new insights into the rational design of advanced electrocatalysts involving coordinated adsorption and activation of multiple reactants.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Hongliu Wan, Lixiang Qian, Nengfeng Gong, Huaming Hou, Xiaomeng Dou, Lirong Zheng, Liang Zhang, Lichen Liu, "Size-Dependent Structures and Catalytic Properties of Supported Bimetallic PtSn Catalysts for Propane Dehydrogenation Reaction", ACS Catalysis, 13, 11, 2023, 7383-7394.
@article{doi:10.1021/acscatal.3c00548,
title = {Size-Dependent Structures and Catalytic Properties of Supported Bimetallic PtSn Catalysts for Propane Dehydrogenation Reaction},
author = {Hongliu Wan and Lixiang Qian and Nengfeng Gong and Huaming Hou and Xiaomeng Dou and Lirong Zheng and Liang Zhang and Lichen Liu},
url = {https://doi.org/10.1021/acscatal.3c00548},
doi = {10.1021/acscatal.3c00548},
year = {2023},
date = {2023-06-02},
urldate = {2023-06-02},
journal = {ACS Catalysis},
volume = {13},
number = {11},
pages = {7383-7394},
abstract = {Heterogeneous bimetallic catalysts are widely used in industrial processes, and the structural features of the bimetallic catalysts have profound impacts on their properties in numerous catalytic processes. Bimetallic nanoclusters with particle sizes ≤1 nm have shown better performances in various catalytic reactions in comparison to conventional bimetallic nanoparticles with sizes above 1 nm. Despite the progress made in recent years in the synthesis and catalytic studies of bimetallic nanoclusters, achieving a fundamental understanding of the structure–reactivity relationships at the molecular and atomic levels remains challenging because of the complexity of the bimetallic catalysts with particle sizes ≤1 nm. In this work, we have studied the structural features of supported bimetallic PtSn species with different sizes (∼0.6 to ∼1.6 nm), which is shown to be associated with the size-dependent formation process of bimetallic PtSn species according to theoretical modeling and experimental studies. Furthermore, the catalytic consequences of their size-dependent structural features are reflected in the dehydrogenation of propane to propylene, in which the subnanometer PtSn clusters are more active than the PtSn alloy nanoparticles.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Hongfei Gu, Wence Yue, Jingqi Hu, Xiangfu Niu, Hao Tang, Fengjuan Qin, You Li, Qing Yan, Xinman Liu, Wenjing Xu, Zhiyi Sun, Qingqing Liu, Wensheng Yan, Lirong Zheng, Yu Wang, Hua Wang, Xinyuan Li, Liang Zhang, Guangming Xia, Wenxing Chen, "Asymmetrically Coordinated Cu–N1C2 Single-Atom Catalyst Immobilized on Ti3C2Tx MXene as Separator Coating for Lithium–Sulfur Batteries", Advanced Energy Materials, 13, 20, 2023, 2204014.
@article{https://doi.org/10.1002/aenm.202204014,
title = {Asymmetrically Coordinated Cu–N1C2 Single-Atom Catalyst Immobilized on Ti3C2Tx MXene as Separator Coating for Lithium–Sulfur Batteries},
author = {Hongfei Gu and Wence Yue and Jingqi Hu and Xiangfu Niu and Hao Tang and Fengjuan Qin and You Li and Qing Yan and Xinman Liu and Wenjing Xu and Zhiyi Sun and Qingqing Liu and Wensheng Yan and Lirong Zheng and Yu Wang and Hua Wang and Xinyuan Li and Liang Zhang and Guangming Xia and Wenxing Chen},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1002/aenm.202204014},
doi = {https://doi.org/10.1002/aenm.202204014},
issn = {1614-6832},
year = {2023},
date = {2023-04-09},
urldate = {2023-04-09},
journal = {Advanced Energy Materials},
volume = {13},
issue = {20},
pages = {2204014},
abstract = {Abstract Lithium?sulfur (Li?S) batteries are receiving great attention owing to their large theoretical energy density, but the shuttle effect and sluggish kinetic conversion of lithium polysulfides (LiPSs) seriously restrict their practical applications. Herein, various metal single-atom catalysts immobilized on nitrogen-doped Ti3C2Tx (M SA/N-Ti3C2Tx, M = Cu, Co, Ni, Mn, Zn, In, Sn, Pb, and Bi) are successfully prepared by a neoteric vacancy-assisted strategy, applied as polypropylene (PP) separator coatings to facilitate the fast redox conversion and adsorption of LiPSs for boosting Li?S batteries. Of particular note, among the M SA/N-Ti3C2Txs, Cu SA/N-Ti3C2Tx/PP exhibits amazing properties, involving excellent rate performance (925 mAh g?1 at 3 C), superb cycling stability over 1000 cycles, and ultra-high sulfur utilization even at large sulfur loadings (7.19 mg cm?2; an areal capacity of 5.28 mAh cm?2). X-ray absorption fine spectroscopy and density functional theory calculations reveal that the asymmetrically coordinated Cu?N1C2 moieties act as the active sites, which possess a higher binding energy and a larger electron cloud with LiPSs than pristine Ti3C2Tx, facilitating the adsorption and kinetic conversion of LiPSs effectively. This work may provide new insights into single atom-decorated ultrathin 2D materials for enhancing electrochemical performance of advanced batteries for energy storage and conversion.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xinyuan Li, Zechao Zhuang, Jing Chai, Ruiwen Shao, Junhui Wang, Zhuoli Jiang, Shuwen Zhu, Hongfei Gu, Jian Zhang, Zhentao Ma, Peng Zhang, Wensheng Yan, Lirong Zheng, Kaifeng Wu, Xusheng Zheng, Liang Zhang, Jiatao Zhang, Dingsheng Wang, Wenxing Chen, Yadong Li, "Atomically Strained Metal Sites for Highly Efficient and Selective Photooxidation", Nano Letters, 23, 7, 2023, 2905-2914.
@article{doi:10.1021/acs.nanolett.3c00256,
title = {Atomically Strained Metal Sites for Highly Efficient and Selective Photooxidation},
author = {Xinyuan Li and Zechao Zhuang and Jing Chai and Ruiwen Shao and Junhui Wang and Zhuoli Jiang and Shuwen Zhu and Hongfei Gu and Jian Zhang and Zhentao Ma and Peng Zhang and Wensheng Yan and Lirong Zheng and Kaifeng Wu and Xusheng Zheng and Liang Zhang and Jiatao Zhang and Dingsheng Wang and Wenxing Chen and Yadong Li},
url = {https://doi.org/10.1021/acs.nanolett.3c00256},
doi = {10.1021/acs.nanolett.3c00256},
issn = {1530-6984},
year = {2023},
date = {2023-03-24},
urldate = {2023-03-24},
journal = {Nano Letters},
volume = {23},
issue = {7},
pages = {2905-2914},
abstract = {Strain engineering is an attractive strategy for improving the intrinsic catalytic performance of heterogeneous catalysts. Manipulating strain on the short-range atomic scale to the local structure of the catalytic sites is still challenging. Herein, we successfully achieved atomic strain modulation on ultrathin layered vanadium oxide nanoribbons by an ingenious intercalation chemistry method. When trace sodium cations were introduced between the V2O5 layers (Na+-V2O5), the V–O bonds were stretched by the atomically strained vanadium sites, redistributing the local charges. The Na+-V2O5 demonstrated excellent photooxidation performance, which was approximately 12 and 14 times higher than that of pristine V2O5 and VO2, respectively. Complementary spectroscopy analysis and theoretical calculations confirmed that the atomically strained Na+-V2O5 had a high surficial charge density, improving the activation of oxygen molecules and contributing to the excellent photocatalytic property. This work provides a new approach for the rational design of strain-equipped catalysts for selective photooxidation reactions.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xin Zhang, Cui Wang, Kai Chen, Adam H. Clark, René Hübner, Jinhua Zhan, Liang Zhang, Alexander Eychmüller, Bin Cai, "Optimizing the Pd Sites in Pure Metallic Aerogels for Efficient Electrocatalytic H2O2 Production", Advanced Materials, 35, 14, 2023, 2211512.
@article{https://doi.org/10.1002/adma.202211512,
title = {Optimizing the Pd Sites in Pure Metallic Aerogels for Efficient Electrocatalytic H2O2 Production},
author = {Xin Zhang and Cui Wang and Kai Chen and Adam H. Clark and René Hübner and Jinhua Zhan and Liang Zhang and Alexander Eychmüller and Bin Cai},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202211512},
doi = {https://doi.org/10.1002/adma.202211512},
issn = {0935-9648},
year = {2023},
date = {2023-02-11},
urldate = {2023-02-11},
journal = {Advanced Materials},
volume = {35},
issue = {14},
pages = {2211512},
abstract = {Decentralized electrochemical production of hydrogen peroxide (H2O2) is an attractive alternative to the industrial anthraquinone process, the application of which is hindered by the lack of high-performance electrocatalysts in acidic media. Herein, a novel catalyst design strategy is reported to optimize the Pd sites in pure metallic aerogels by tuning their geometric environments and electronic structures. By increasing the Hg content in the Pd–Hg aerogels, the Pd-Pd coordination is gradually diminished, resulting in isolated, single-atom-like Pd motifs in the Pd2Hg5 aerogel. Further heterometal doping leads to a series of M–Pd2Hg5 aerogels with an unalterable geometric environment, allowing for sole investigation of the electronic effects. Combining theoretical and experimental analyses, a volcano relationship is obtained for the M–Pd2Hg5 aerogels, demonstrating an effective tunability of the electronic structure of the Pd active sites. The optimized Au–Pd2Hg5 aerogel exhibits an outstanding H2O2 selectivity of 92.8% as well as transferred electron numbers of ≈2.1 in the potential range of 0.0–0.4 VRHE. This work opens a door for designing metallic aerogel electrocatalysts for H2O2 production and highlights the importance of electronic effects in tuning electrocatalytic performances.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xing Li, Shaobo Cheng, Yanghua He, Lixiang Qian, Dmitri Zakharov, Gang Wu, Chongxin Shan, Liang Zhang, Dong Su, "Revealing the dynamics of the alloying and segregation of Pt-Co nanoparticles via in-situ environmental transmission electron microscopy", Nano Research, 16, 2, 2023, 3055-3062.
@article{,
title = {Revealing the dynamics of the alloying and segregation of Pt-Co nanoparticles via in-situ environmental transmission electron microscopy},
author = {Xing Li and Shaobo Cheng and Yanghua He and Lixiang Qian and Dmitri Zakharov and Gang Wu and Chongxin Shan and Liang Zhang and Dong Su},
url = {https://www.sciopen.com/article/10.1007/s12274-022-5012-0},
doi = {10.1007/s12274-022-5012-0},
issn = {1998-0000},
year = {2023},
date = {2023-02-01},
urldate = {2022-11-05},
journal = {Nano Research},
volume = {16},
issue = {2},
pages = {3055-3062},
abstract = {Thermal treatment is a general and efficient way to synthesize intermetallic catalysts and may involve complicated physical processes. So far, the mechanisms leading to the size and composition heterogeneity, as well as the phase segregation behavior in Pt-Co nanoparticles (NPs) are still not well understood. Via in-situ environmental transmission electron microscopy, the formation dynamics and segregation behaviors of Pt-Co alloyed NPs during the thermal treatment were investigated. It is found that Pt-Co NPs on zeolitic imidazolate frameworks-67-derived nanocarbon (NC) are formed consecutively through both particle migration coalescence and the Ostwald ripening process. The existence of Pt NPs is found to affect the movement of Co NPs during their migration. With the help of theoretical calculations, the correlations between the composition and migration of the Pt and Co during the ripening process were uncovered. These complex alloying processes are revealed as key factors leading to the heterogeneity of the synthesized Pt-Co alloyed NPs. Under oxidation environment, the Pt-Co NPs become surface faceted gradually, which can be attributed to the oxygen facilitated relatively higher segregation rate of Co from the (111) surface. This work advances the fundamental understanding of design, synthesis, and durability of the Pt-based nanocatalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2022
Shuyan Gong, Mingze Sun, Yi-Yang Lee, Nigel Becknell, Jiangwei Zhang, Zhongqi Wang, Liang Zhang, Zhiqiang Niu, "Bulk-like Pt(100)-oriented Ultrathin Surface: Combining the Merits of Single Crystals and Nanoparticles to Boost Oxygen Reduction Reaction", Angewandte Chemie International Edition, 62, 4, 2022, e202214516.
@article{https://doi.org/10.1002/anie.202214516,
title = {Bulk-like Pt(100)-oriented Ultrathin Surface: Combining the Merits of Single Crystals and Nanoparticles to Boost Oxygen Reduction Reaction},
author = {Shuyan Gong and Mingze Sun and Yi-Yang Lee and Nigel Becknell and Jiangwei Zhang and Zhongqi Wang and Liang Zhang and Zhiqiang Niu},
url = {https://onlinelibrary.wiley.com/doi/epdf/10.1002/anie.202214516
https://onlinelibrary.wiley.com/action/downloadSupplement?doi=10.1002%2Fanie.202214516&file=anie202214516-s1-supporting_information.pdf},
doi = {https://doi.org/10.1002/anie.202214516},
issn = {1433-7851},
year = {2022},
date = {2022-11-24},
urldate = {2022-11-24},
journal = {Angewandte Chemie International Edition},
volume = {62},
number = {4},
pages = {e202214516},
abstract = {Single crystal surfaces with highly coordinated sites very often hold high specific activities toward oxygen reduction reaction (ORR) and others. Transposing their high specific activity to practical high-surface-area electrocatalysts remains challenging. Here, ultrathin Pt(100) alloy surface is constructed via epitaxial growth. The surface shows 3.1‒6.9% compressive strain and bulk-like characteristics as demonstrated by site-probe reactions and different spectroscopies. Its ORR activity exceeds that of bulk Pt3Ni(100) and Pt(111) and presents a 19-fold increase in specific activity and a 13-fold increase in mass activity relative to commercial Pt/C. Moreover, the electrochemically active surface area (ECSA) is increased by 4-fold compared to traditional thin films (e.g. NSTF), which makes the catalyst more tolerant to voltage loss at high current densities under fuel cell operation. This work broadens the family of extended surface catalysts and highlights the knowledge-driven approach in the development of advanced electrocatalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Tingting Chao, Xuan Luo, Mengzhao Zhu, Yanmin Hu, Yida Zhang, Yunteng Qu, Hantao Peng, Xiaoshuang Shen, Xusheng Zheng, Liang Zhang, Xun Hong, "The Promoting Effect of Interstitial Hydrogen on the Oxygen Reduction Performance of PtPd Alloy Nanotubes for Fuel Cells", Nano Research, 16, 2, 2022, 2366-2372.
@article{Chao2023,
title = {The Promoting Effect of Interstitial Hydrogen on the Oxygen Reduction Performance of PtPd Alloy Nanotubes for Fuel Cells},
author = {Tingting Chao and Xuan Luo and Mengzhao Zhu and Yanmin Hu and Yida Zhang and Yunteng Qu and Hantao Peng and Xiaoshuang Shen and Xusheng Zheng and Liang Zhang and Xun Hong},
url = {https://www.sciopen.com/article/10.1007/s12274-022-4891-4},
doi = {10.1007/s12274-022-4891-4},
issn = {1998-0124},
year = {2022},
date = {2022-08-30},
urldate = {2022-08-10},
journal = {Nano Research},
volume = {16},
issue = {2},
pages = {2366-2372},
abstract = {Highly efficient and stable oxygen reduction reaction (ORR) electrocatalysts are remarkably important but challenging for advancing the large-scale commercialization of practical proton exchange membrane fuel cells (PEMFCs). In this work, we report that the introduction of interstitial hydrogen atoms into PtPd nanotubes can significantly promote ORR performance without scarifying the durability. The enhanced mass activity was 8.8 times higher than that of commercial Pt/C. The accelerated durability test showed negligible activity attenuation after 30,000 cycles. Additionally, H2/O2 fuel cell tests further verified the excellent activity of PtPd-H nanotubes with a maximum power density of 1.32 W cm-2, superior to that of commercial Pt/C (1.16 W cm-2). Density functional theory calculations demonstrates the incorporation of hydrogen atoms gives rise to the broadening of Pt d-band and the downshift of d-band center, which consequently leads to the weaker intermediates binding and enhanced ORR activity.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xin Geng, Shuwei Li, Zhi Mei, Dongsheng Li, Liang Zhang, Long Luo, "Ultrafast metal oxide reduction at Pd/PdO2 interface enables one-second hydrogen gas detection under ambient conditions", Nano Research, 16, 1, 2022, 1149-1157.
@article{Geng2023,
title = {Ultrafast metal oxide reduction at Pd/PdO2 interface enables one-second hydrogen gas detection under ambient conditions},
author = {Xin Geng and Shuwei Li and Zhi Mei and Dongsheng Li and Liang Zhang and Long Luo},
url = {https://www.sciopen.com/article/10.1007/s12274-022-4816-2},
doi = {10.1007/s12274-022-4816-2},
issn = {1998-0124},
year = {2022},
date = {2022-08-19},
urldate = {2022-07-26},
journal = {Nano Research},
volume = {16},
issue = {1},
pages = {1149-1157},
abstract = {Here, we report a Pd/PdOx sensing material that achieves 1-s detection of 4% H2 gas (i.e., the lower explosive limit concentration for H2) at room temperature in air. The Pd/PdOx material is a network of interconnected nanoscopic domains of Pd, PdO, and PdO2. Upon exposure to 4% H2, PdO and PdO2 in the Pd/PdOx are immediately reduced to metallic Pd, generating over a >90% drop in electrical resistance. The mechanistic study reveals that the Pd/PdO2 interface in Pd/PdOx is responsible for the ultrafast PdOx reduction. Metallic Pd at the Pd/PdO2 interface enables fast H2 dissociation to adsorbed H atoms, significantly lowering the PdO2 reduction barrier. In control
suggest that the interconnectivity of Pd, PdO, and PdO2 in the our Pd/PdOx sensing material further the of PdO, which would not occur. The 1-s response time of Pd/PdOx under ambient conditions makes it an excellent alarm for the timely detection of hydrogen gas leaks.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
suggest that the interconnectivity of Pd, PdO, and PdO2 in the our Pd/PdOx sensing material further the of PdO, which would not occur. The 1-s response time of Pd/PdOx under ambient conditions makes it an excellent alarm for the timely detection of hydrogen gas leaks.
Xin Geng, Shuwei Li, Jaeyoung Heo, Yi Peng, Wenhui Hu, Yanchao Liu, Jier Huang, Yang Ren, Dongsheng Li, Liang Zhang, Long Luo, "Grain-Boundary-Rich Noble Metal Nanoparticle Assemblies: Synthesis, Characterization, and Reactivity", Advanced Functional Materials, 2022, 2204169.
@article{geng2022grain,
title = {Grain-Boundary-Rich Noble Metal Nanoparticle Assemblies: Synthesis, Characterization, and Reactivity},
author = {Xin Geng and Shuwei Li and Jaeyoung Heo and Yi Peng and Wenhui Hu and Yanchao Liu and Jier Huang and Yang Ren and Dongsheng Li and Liang Zhang and Long Luo},
url = {https://onlinelibrary.wiley.com/doi/epdf/10.1002/adfm.202204169},
doi = {10.1002/adfm.202204169},
year = {2022},
date = {2022-06-15},
journal = {Advanced Functional Materials},
pages = {2204169},
publisher = {Wiley Online Library},
abstract = { },
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xinbin Wu, Wei Yu, Wei Xu, Yujun Zhang, Shundong Guan, Zheng Zhang, Shuwei Li, Huanchun Wang, Xuanjun Wang, Liang Zhang, Ce-Wen Nan, Liangliang Li, "Balancing oxygen evolution reaction and oxygen reduction reaction processes in Li–O2 batteries through tuning the bond distances of RuO2", Composites Part B: Engineering, 234, 2022, 109727.
@article{WU2022109727,
title = {Balancing oxygen evolution reaction and oxygen reduction reaction processes in Li–O2 batteries through tuning the bond distances of RuO2},
author = {Xinbin Wu and Wei Yu and Wei Xu and Yujun Zhang and Shundong Guan and Zheng Zhang and Shuwei Li and Huanchun Wang and Xuanjun Wang and Liang Zhang and Ce-Wen Nan and Liangliang Li},
url = {https://www.sciencedirect.com/science/article/pii/S1359836822001123},
doi = {https://doi.org/10.1016/j.compositesb.2022.109727},
issn = {1359-8368},
year = {2022},
date = {2022-04-01},
journal = {Composites Part B: Engineering},
volume = {234},
pages = {109727},
abstract = {Lithium-oxygen (Li–O2) batteries are promising next-generation energy storage devices due to their ultrahigh theoretical specific energy. Oxide electrocatalysts such as RuO2 are often used to improve the oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) processes in Li–O2 batteries, but the relation between the structural parameters and their catalytic properties is unclear so far. In this work, we synthesize RuO2 nanoparticles at different annealing temperatures and investigate the influence of the Ru–O bond distances of RuO2 on the OER and ORR processes in Li–O2 batteries. The results of X-ray absorption fine structure show that the bond distances decrease with the increase of the annealing temperature. The RuO2 with longer Ru–O bond distances renders a lower charge plateau to Li–O2 batteries, showing a better OER performance, while that with shorter bond distances gives a larger discharge capacity and is more friendly to the ORR process. The cycle life of Li–O2 batteries with a carbon nanotube-RuO2 composite cathode is improved to 287 cycles (1148 h) under an extreme testing condition (the voltage range is 2.3–4.2 V), when the Ru–O bond distances are optimized. In addition, the influence of the Ru–O bond distances on the catalytic properties of RuO2 is analyzed by density functional theory calculations. The findings in this work provide a guidance to enhance the ORR and OER efficiency of RuO2 and other oxide catalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2021
Xin Geng, Xiaolong Liu, Lalani Mawella-Vithanage, Chathuranga C Hewa-Rahinduwage, Liang Zhang, Stephanie L Brock, Ting Tan, Long Luo, "Photoexcited NO2 Enables Accelerated Response and Recovery Kinetics in Light-Activated NO2 Gas Sensing", ACS Sensors, 0, 0, 2021, null.
@article{doi:10.1021/acssensors.1c01694c,
title = {Photoexcited NO2 Enables Accelerated Response and Recovery Kinetics in Light-Activated NO2 Gas Sensing},
author = {Xin Geng and Xiaolong Liu and Lalani Mawella-Vithanage and Chathuranga C Hewa-Rahinduwage and Liang Zhang and Stephanie L Brock and Ting Tan and Long Luo},
url = {https://zhanglab-thu.com/wp-content/uploads/2021/11/acssensors.1c01694.pdf},
doi = {10.1021/acssensors.1c01694},
year = {2021},
date = {2021-11-30},
journal = {ACS Sensors},
volume = {0},
number = {0},
pages = {null},
abstract = {Slow response and recovery kinetics is a major challenge for practical room-temperature NO2 gas sensing. Here, we report the use of visible light illumination to significantly shorten the response and recovery times of a PbSe quantum dot (QD) gel sensor by 21% (to 27 s) and 63% (to 102 s), respectively. When combined with its high response (0.04%/ppb) and ultralow limit of detection (3 ppb), the reduction in response and recovery time makes the PbSe QD gel sensor among the best p-type room-temperature NO2 sensors reported to date. A combined experimental and theoretical investigation reveals that the accelerated response and recovery time is primarily due to photoexcitation of NO2 gaseous molecules and adsorbed NO2 on the gel surface, rather than the excitation of the semiconductor sensing material, as suggested by the currently prevailing light-activated gas-sensing theory. Furthermore, we find that the extent of improvement attained in the recovery speed also depends on the distribution of excited electrons in the adsorbed NO2/QD gel system. This work suggests that the design of light-activated sensor platforms may benefit from a careful assessment of the photophysics of the analyte in the gas phase and when adsorbed onto the semiconductor surface.},
note = {PMID: 34784175},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
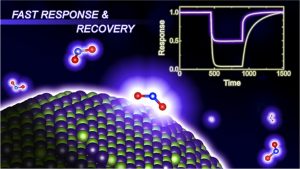
Xin Geng, Shuwei Li, Lalani Mawella-Vithanage, Tao Ma, Mohamed Kilani, Bingwen Wang, Lu Ma, Chathuranga C Hewa-Rahinduwage, Alina Shafikova, Eranda Nikolla, Guangzhao Mao, Stephanie L. Brock, Liang Zhang, Long Luo, "Atomically dispersed Pb ionic sites in PbCdSe quantum dot gels enhance room-temperature NO2 sensing", Nature communications, 12, 1, 2021, 1-11.
@article{geng2021atomically,
title = {Atomically dispersed Pb ionic sites in PbCdSe quantum dot gels enhance room-temperature NO2 sensing},
author = {Xin Geng and Shuwei Li and Lalani Mawella-Vithanage and Tao Ma and Mohamed Kilani and Bingwen Wang and Lu Ma and Chathuranga C Hewa-Rahinduwage and Alina Shafikova and Eranda Nikolla and Guangzhao Mao and Stephanie L. Brock and Liang Zhang and Long Luo},
url = {https://www.nature.com/articles/s41467-021-25192-4.pdf, PDF},
doi = {10.1038/s41467-021-25192-4},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Nature communications},
volume = {12},
number = {1},
pages = {1-11},
publisher = {Nature Publishing Group},
abstract = {Atmospheric NO2 is of great concern due to its adverse effects on human health and the environment, motivating research on NO2 detection and remediation. Existing low-cost room-temperature NO2 sensors often suffer from low sensitivity at the ppb level or long recovery times, reflecting the trade-off between sensor response and recovery time. Here, we report an atomically dispersed metal ion strategy to address it. We discover that bimetallic PbCdSe quantum dot (QD) gels containing atomically dispersed Pb ionic sites achieve the optimal combination of strong sensor response and fast recovery, leading to a high-performance room-temperature p-type semiconductor NO2 sensor as characterized by a combination of ultra–low limit of detection, high sensitivity and stability, fast response and recovery. With the help of theoretical calculations, we reveal the high performance of the PbCdSe QD gel arises from the unique tuning effects of Pb ionic sites on NO2 binding at their neighboring Cd sites.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Chenhui Zhou, Siming Zhao, Haibing Meng, Ying Han, Qinyuan Jiang, Baoshun Wang, Xiaofei Shi, Wenshuo Zhang, Liang Zhang, Rufan Zhang, "RuCoOx Nanofoam as a High-Performance Trifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries and Water Splitting", Nano Letters, 21, 22, 2021, 9633-9641.
@article{doi:10.1021/acs.nanolett.1c03407,
title = {RuCoOx Nanofoam as a High-Performance Trifunctional Electrocatalyst for Rechargeable Zinc–Air Batteries and Water Splitting},
author = {Chenhui Zhou and Siming Zhao and Haibing Meng and Ying Han and Qinyuan Jiang and Baoshun Wang and Xiaofei Shi and Wenshuo Zhang and Liang Zhang and Rufan Zhang},
url = {https://zhanglab-thu.com/wp-content/uploads/2021/11/acs.nanolett.1c03407.pdf},
doi = {10.1021/acs.nanolett.1c03407},
year = {2021},
date = {2021-01-01},
journal = {Nano Letters},
volume = {21},
number = {22},
pages = {9633-9641},
abstract = {Designing high-performance trifunctional electrocatalysts for ORR/OER/HER with outstanding activity and stability for each reaction is quite significant yet challenging for renewable energy technologies. Herein, a highly efficient and durable trifunctional electrocatalyst RuCoOx is prepared by a unique one-pot glucose-blowing approach. Remarkably, RuCoOx catalyst exhibits a small potential difference (ΔE) of 0.65 V and low HER overpotential of 37 mV (10 mA cm–2), as well as a negligible decay of overpotential after 200 000/10 000/10 000 CV cycles for ORR/OER/HER, all of which show overwhelming superiorities among the advanced trifunctional electrocatalysts. When used in liquid rechargeable Zn–air batteries and water splitting electrolyzer, RuCoOx exhibits high efficiency and outstanding durability even at quite large current density. Such excellent performance can be attributed to the rational combination of targeted ORR/OER/HER active sites into one electrocatalyst based on the double-phase coupling strategy, which induces sufficient electronic structure modulation and synergistic effect for enhanced trifunctional properties.},
note = {PMID: 34761938},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

2020
Liang Zhang, May Ling Ng, Aleksandra Vojvodic, "Role of Undercoordinated Sites for the Catalysis in Confined Spaces Formed by Two-Dimensional Material Overlayers", The Journal of Physical Chemistry Letters, 11, 21, 2020, 9400-9407.
@article{Zhang2020bb,
title = {Role of Undercoordinated Sites for the Catalysis in Confined Spaces Formed by Two-Dimensional Material Overlayers},
author = {Liang Zhang and May Ling Ng and Aleksandra Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.jpclett.0c02652.pdf, PDF},
doi = {10.1021/acs.jpclett.0c02652},
issn = {1948-7185},
year = {2020},
date = {2020-11-01},
urldate = {2020-11-01},
journal = {The Journal of Physical Chemistry Letters},
volume = {11},
number = {21},
pages = {9400--9407},
abstract = {Adding a two-dimensional (2D) overlayer on a metal surface is a promising route for activating reactants confined in the interfacial space. However, an atomistic understanding of the role played by undercoordinated sites of the 2D overlayer in the activation of molecules in this nanoscaled confined space is yet to be developed. In this paper, we study CO dissociation as a prototypical reaction to investigate CO activation in the confined space enclosed by Rh(111) and a monolayer of hexagonal boron nitride (h-BN). The effect of the space size (i.e., the distance between h-BN and the metal surface), the type of undercoordinated sites, and the size of the defect are explicitly studied by density functional theory with dispersion correction. The following temperature-programmed X-ray photoelectron spectroscopy measurement suggests that a small portion of the CO dissociated during the desorption, leaving the residual atomic oxygen incorporated into the h-BN lattice, which validates the theoretical prediction. The combination of theory and experiment calls for further attention to be paid to the role of undercoordinated sites in the 2D overlayers in confined systems forming potential new catalytic environments.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Chathuranga C Hewa-Rahinduwage, Xin Geng, Karunamuni L Silva, Xiangfu Niu, Liang Zhang, Stephanie L Brock, Long Luo, "Reversible Electrochemical Gelation of Metal Chalcogenide Quantum Dots", Journal of the American Chemical Society, 142, 28, 2020, 12207-12215.
@article{Hewa-Rahinduwage2020,
title = {Reversible Electrochemical Gelation of Metal Chalcogenide Quantum Dots},
author = {Chathuranga C Hewa-Rahinduwage and Xin Geng and Karunamuni L Silva and Xiangfu Niu and Liang Zhang and Stephanie L Brock and Long Luo},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/jacs.0c03156.pdf, PDF},
doi = {10.1021/jacs.0c03156},
issn = {0002-7863},
year = {2020},
date = {2020-07-01},
journal = {Journal of the American Chemical Society},
volume = {142},
number = {28},
pages = {12207--12215},
abstract = {The ability to dictate the assembly of quantum dots (QDs) is critical for their integration into solid-state electronic and optoelectronic devices. However, assembly methods that enable efficient electronic communication between QDs, facilitate access to the reactive surface, and retain the native quantum confinement characteristics of the QD are lacking. Here we introduce a universal and facile electrochemical gelation method for assembling metal chalcogenide QDs (as demonstrated for CdS, ZnS, and CdSe) into macroscale 3-D connected pore-matter nanoarchitectures that remain quantum confined and in which each QD is accessible to the ambient. Because of the redox-active nature of the bonding between QD building blocks in the gel network, the electrogelation process is reversible. We further demonstrate the application of this electrogelation method for a one-step fabrication of CdS gel gas sensors, producing devices with exceptional performance for NO2 gas sensing at room temperature, thereby enabling the development of low-cost, sensitive, and reliable devices for air quality monitoring.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Liang Zhang, Abhinav S Raman, Aleksandra Vojvodic, "Reviving Inert Oxides for Electrochemical Water Splitting by Subsurface Engineering", Chemistry of Materials, 32, 13, 2020, 5569-5578.
@article{Zhang2020a,
title = {Reviving Inert Oxides for Electrochemical Water Splitting by Subsurface Engineering},
author = {Liang Zhang and Abhinav S Raman and Aleksandra Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.chemmater.0c00763.pdf, PDF},
doi = {10.1021/acs.chemmater.0c00763},
issn = {0897-4756},
year = {2020},
date = {2020-07-01},
urldate = {2020-07-01},
journal = {Chemistry of Materials},
volume = {32},
number = {13},
pages = {5569--5578},
abstract = {Recently, it was theoretically predicted and experimentally validated that subsurface alloying of SrRuO3 (SRO) beneath the SrTiO3 (STO) capping layer can significantly promote the otherwise inert STO surface toward oxygen evolution [ Akbashev et al. Energy Environ. Sci. 2018, 11, 1762-1769[. Herein, we provide a generalized framework behind the concept of subsurface alloying with different transition-metal dopants, host metal oxides, and doping levels. Based on density functional theory (DFT) calculations and detailed electronic-structure analysis, we first identify the electronic structure origin of the activation and stabilization phenomena and propose a tuning mechanism that enables the identification of candidate subsurface dopants in STO, with the highest activity for both oxygen and hydrogen evolution reactions. We then show that the proposed mechanism is applicable to subsurface alloys formed with other host materials such as SrZrO3, TiO2, and ZrO2. Finally, we propose a materials design scheme using partial subsurface alloying for more precise tuning of surface reactivity and activity. By generalizing the concept of subsurface alloying of metal oxides, our work explains why the SRO subsurface alloyed STO has among the highest OER enhancements and importantly provides a new route in tailoring the activity and stability of earth-abundant electrocatalysts for water splitting.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Yongchen Wang, Shutang Chen, Xudong Wang, Adam Rosen, William Beatrez, Lukasz Sztaberek, Haiyan Tan, Liang Zhang, Christopher Koenigsmann, Jing Zhao, "Composition-Dependent Oxygen Reduction Reaction Activity of Pt-Surfaced PtNi Dodecahedral Nanoframes", ACS Applied Energy Materials, 3, 1, 2020, 768-776.
@article{Wang2020,
title = {Composition-Dependent Oxygen Reduction Reaction Activity of Pt-Surfaced PtNi Dodecahedral Nanoframes},
author = {Yongchen Wang and Shutang Chen and Xudong Wang and Adam Rosen and William Beatrez and Lukasz Sztaberek and Haiyan Tan and Liang Zhang and Christopher Koenigsmann and Jing Zhao},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acsaem.9b01930.pdf, PDF},
doi = {10.1021/acsaem.9b01930},
issn = {2574-0962},
year = {2020},
date = {2020-01-01},
journal = {ACS Applied Energy Materials},
volume = {3},
number = {1},
pages = {768--776},
abstract = {Pt-based bimetallic nanoframes have been demonstrated to have high activity for a number of electrocatalytic reactions. Their morphology, crystal facets, and compositions are important factors that regulate their catalytic activities. Herein, we synthesized a series of Pt-surfaced PtNi dodecahedral nanoframes with variable Pt/Ni ratios. The nanoframes were prepared by oxidative etching of presynthesized PtNi rhombic dodecahedron nanoparticles. The Pt ratio in the PtNi nanoframes have been tuned from 28% to 65% by changing the duration of oxidative etching. In terms of catalytic performance, the PtNi nanoframes display a volcano-type behavior in specific oxygen reduction reaction (ORR) activity as a function of Pt ratio with a maximum ORR specific activity of 1.9 mA cm-2 observed with 47% Pt. The mass activity of the particles ranges from 0.72 to 0.92 A mg-1, which significantly exceeds the mass activity of 0.19 A mg-1 measured for commercial Pt NP/C. Density functional theory calculations reveal that the Pt ratio underneath the Pt skin in the nanoframes affects the binding energy of oxygenate species and thus the ORR activity. The trend of OH binding energy versus PtNi composition from the computational results qualitatively agrees with the trend of ORR activity from the experiments.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Luke R Johnson, Sudiksha Sridhar, Liang Zhang, Kurt D Fredrickson, Abhinav S Raman, Joonbaek Jang, Connor Leach, Ashwin Padmanabhan, Christopher C Price, Nathan C Frey, Abhishek Raizada, Vishwanathan Rajaraman, Sai Aparna Saiprasad, Xiaoxin Tang, Aleksandra Vojvodic, "MXene Materials for the Electrochemical Nitrogen Reduction—Functionalized or Not?", ACS Catalysis, 10, 1, 2020, 253-264.
@article{Johnson2020,
title = {MXene Materials for the Electrochemical Nitrogen Reduction—Functionalized or Not?},
author = {Luke R Johnson and Sudiksha Sridhar and Liang Zhang and Kurt D Fredrickson and Abhinav S Raman and Joonbaek Jang and Connor Leach and Ashwin Padmanabhan and Christopher C Price and Nathan C Frey and Abhishek Raizada and Vishwanathan Rajaraman and Sai Aparna Saiprasad and Xiaoxin Tang and Aleksandra Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acscatal.9b01925.pdf, PDF},
doi = {10.1021/acscatal.9b01925},
issn = {2155-5435},
year = {2020},
date = {2020-01-01},
journal = {ACS Catalysis},
volume = {10},
number = {1},
pages = {253--264},
abstract = {We use density functional theory calculations to study a group of 2D materials known as MXenes toward the electrochemical nitrogen reduction reaction (NRR) to ammonia. So far, all computational studies have only considered the NRR chemistry on unfunctionalized (bare) MXenes. In this study, we investigate a total of 65 bare and functionalized MXenes. We establish free energy diagrams for the NRR on the basal planes of 55 different M2XTx MXenes (M = Ti, V, Zr, Nb, Mo, Ta, W; X = C, N) to span a large variety of possible chemistries. Energy trends with respect to the metal as well as nonmetal constituent of the MXenes are established for both bare and functionalized MXenes. We determine the limiting potentials and find that either the formation of NH3 from*NH2 or the formation of*N2H is the potential limiting reaction step for bare and functionalized MXenes, respectively. We find several Mo-, W-, and V-based MXenes (Mo2C, Mo2N, W2N, W2NH2, and V2N) to have suitable theoretical overpotentials for the NRR. Importantly, calculated Pourbaix stability diagrams combined with selectivity analysis, however, reveal that all bare MXenes are not stable under relevant NRR operating conditions. The only functionalized MXene with the three minimum required properties (i) having a low theoretical overpotential, (ii) being stable under NRR conditions, and (iii) having selectivity toward NRR rather than the parasitic HER is W2CH2, which is a H-Terminated MXene. Finally, on the basis of our findings, we explore other routes for improving the NRR chemistry by studying 10 additional MXenes with the chemical formula M3X2Tx and MXenes with other functional groups (Tx = S, F, Cl). This opens up a larger variety and tunability of MXenes to be considered for the NRR.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Ran Sui, Wenkai Liang, Liang Zhang, John Mantzaras, Chung K Law, "Kinetic interactions between H2 and CO in catalytic oxidation over PdO", Combustion and Flame, 211, 2020, 270-280.
@article{Sui2020,
title = {Kinetic interactions between H2 and CO in catalytic oxidation over PdO},
author = {Ran Sui and Wenkai Liang and Liang Zhang and John Mantzaras and Chung K Law},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/1-s2.0-S0010218019304493-main.pdf, PDF},
doi = {10.1016/j.combustflame.2019.09.035},
issn = {00102180},
year = {2020},
date = {2020-01-01},
journal = {Combustion and Flame},
volume = {211},
pages = {270--280},
abstract = {Kinetic interactions between H2 and CO over PdO, a widely used catalyst in combustion systems, were studied experimentally and numerically. Global reaction parameters of H2 and CO oxidation over PdO were extracted from wire microcalorimetry experiments at atmospheric pressure in the temperature range 380–800 K, based on which a full catalytic reaction mechanism was developed. Comparison of ignition temperatures and heat release rates of different H2/CO blends along with density functional theory (DFT) simulations revealed complex physicochemical coupling of the H2 and CO catalytic oxidation pathways. The coupling evolves from an inhibiting effect of one fuel component onto the other due to their competition for surface adsorption sites and a direct repelling mechanism between the co-adsorbed H(s) and CO(s), to a promoting effect at sufficiently high temperatures caused by alleviated O(s) surface blocking. Implications of the H2[sbnd]CO kinetic coupling to the operation of practical power generation systems are outlined.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xue Zhang, Jian Han, Xiangfu Niu, Chengzhou Xin, Chuanjiao Xue, Shuo Wang, Yang Shen, Liang Zhang, Liangliang Li, Ce‐Wen Nan, "High Cycling Stability for Solid‐State Li Metal Batteries via Regulating Solvation Effect in Poly(Vinylidene Fluoride)‐Based Electrolytes", Batteries & Supercaps, 2020.
@article{Zhang2020b,
title = {High Cycling Stability for Solid‐State Li Metal Batteries via Regulating Solvation Effect in Poly(Vinylidene Fluoride)‐Based Electrolytes},
author = {Xue Zhang and Jian Han and Xiangfu Niu and Chengzhou Xin and Chuanjiao Xue and Shuo Wang and Yang Shen and Liang Zhang and Liangliang Li and Ce‐Wen Nan},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/batt.202000081.pdf, PDF},
doi = {10.1002/batt.202000081},
issn = {2566-6223},
year = {2020},
date = {2020-01-01},
journal = {Batteries & Supercaps},
abstract = {Solid polymer electrolytes have emerged as promising alternatives to current liquid electrolytes due to their advantages in battery safety and stability. Among various polymer electrolytes, poly(vinylidene fluoride) (PVDF)-based electrolytes with high ionic conductivity, large mechanical strength, and excellent electrochemical and thermal stability have a great potential for practical applications. However, fundamental issues, such as how the Li ions transport in the PVDF-based electrolytes and how the residual solvent affects the cell performance, are unclear. Here, we demonstrate that the solvation effect due to a small amount of residual N,N-dimethylformamide (DMF) bound into the electrolytes plays a
critical role in ionic transport, interface stability, and cell performance. With the residual DMF existing in the electrolytes in a bound state not as free solvent, the ionic conduction could be realized by the Li-ion transport among the interaction sites between the bound DMF and PVDF chains. Regulating the solvation effect in the electrolytes can make the PVDF-based solid-state Li metal batteries a significantly improved cycling performance at 25 degrees C (e. g., over 1000 cycles with a capacity retention of more than 94 %). These findings would promote the development of next-generation Li metal batteries with high energy density and safety.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
critical role in ionic transport, interface stability, and cell performance. With the residual DMF existing in the electrolytes in a bound state not as free solvent, the ionic conduction could be realized by the Li-ion transport among the interaction sites between the bound DMF and PVDF chains. Regulating the solvation effect in the electrolytes can make the PVDF-based solid-state Li metal batteries a significantly improved cycling performance at 25 degrees C (e. g., over 1000 cycles with a capacity retention of more than 94 %). These findings would promote the development of next-generation Li metal batteries with high energy density and safety.

2019
Anthony Curto, Zhaozong Sun, Jonathan Rodríguez-Fernández, Liang Zhang, Ayush Parikh, Ting Tan, Jeppe V Lauritsen, Aleksandra Vojvodic, "Anisotropic iron-doping patterns in two-dimensional cobalt oxide nanoislands on Au(111)", Nano Research, 12, 9, 2019, 2364-2372.
@article{Curto2019,
title = {Anisotropic iron-doping patterns in two-dimensional cobalt oxide nanoislands on Au(111)},
author = {Anthony Curto and Zhaozong Sun and Jonathan Rodríguez-Fernández and Liang Zhang and Ayush Parikh and Ting Tan and Jeppe V Lauritsen and Aleksandra Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/Curto2019_Article_AnisotropicIron-dopingPatterns.pdf, PDF},
doi = {10.1007/s12274-019-2474-9},
issn = {1998-0124},
year = {2019},
date = {2019-09-01},
journal = {Nano Research},
volume = {12},
number = {9},
pages = {2364--2372},
abstract = {An integrated approach combining density functional theory (DFT) calculations and atomic resolution scanning tunneling microscopy (STM) is used to study well-defined iron-doped cobalt oxide nanoislands supported on Au(111). The focus is on the structure and distribution of Fe dopants within these nanoislands of CoO as a function of Fe to Co ratio. The DFT and STM results agree strongly and complement each other to allow for a more complete understanding of the dopant structure trends on the nanoscale. Using Fe as a marker, we first find that the stacking sequence of the moiré structure of the host cobalt oxide nanoislands can be identified unambiguously through a combination of DFT and STM. Using the distinct contrast of the embedded Fe dopant atoms as observed with atom-resolved STM, we find correlations between Fe dopant position and the CoO/Au(111) moiré pattern at varying Fe dopant densities. Formation of Fe-dopant clusters within the nanoislands is investigated in detail through DFT and found to agree with the dopant patterns observed in STM. We find that the structural effects of Fe dopants throughout the nanoislands with the basal planes and the two types of edges—the oxygen and metal edges—have different nature. Both DFT calculations and STM images show a strong preference for Fe dopants to be located directly on or near the oxygen edge of the nanoislands as opposed to being directly on or near the metal edge. Taken together, our results illustrate that Fe dopant incorporation and distribution within CoO nanoislands are highly anisotropic and governed by both the moiré structure of the basal planes as well as nano-size effects present at the under-coordinated edges of different local geometry and chemistries. [Figure not available: see fulltext.]},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
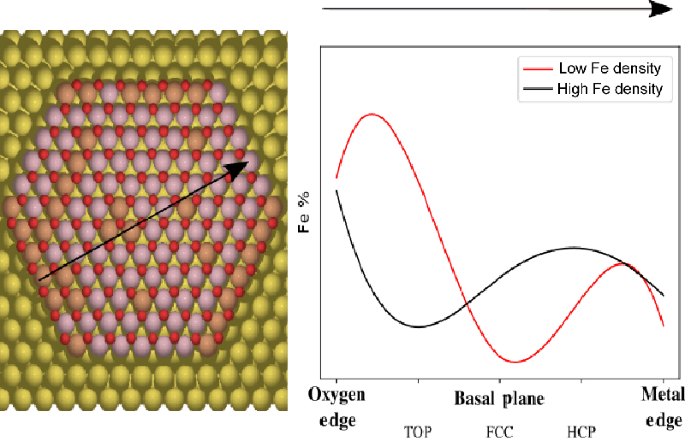
Yuan Cheng, Abhinav S Raman, Julian Paige, Liang Zhang, Danyi Sun, Mavis U Chen, Aleksandra Vojvodic, Raymond J Gorte, John M Vohs, "Enhancing Oxygen Exchange Activity by Tailoring Perovskite Surfaces", The Journal of Physical Chemistry Letters, 10, 14, 2019, 4082-4088.
@article{Cheng2019,
title = {Enhancing Oxygen Exchange Activity by Tailoring Perovskite Surfaces},
author = {Yuan Cheng and Abhinav S Raman and Julian Paige and Liang Zhang and Danyi Sun and Mavis U Chen and Aleksandra Vojvodic and Raymond J Gorte and John M Vohs},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.jpclett.9b01235.pdf, PDF},
doi = {10.1021/acs.jpclett.9b01235},
issn = {1948-7185},
year = {2019},
date = {2019-07-01},
journal = {The Journal of Physical Chemistry Letters},
volume = {10},
number = {14},
pages = {4082--4088},
abstract = {A detailed understanding of the effects of surface chemical and geometric composition is essential for understanding the electrochemical performance of the perovskite (ABO3) oxides commonly used as electrocatalysts in the cathodes of ceramic fuel cells. Herein, we report how the addition of submonolayer quantities of A- and B-site cations affects the rate of the oxygen reduction reaction (ORR) of Sr-doped LaFeO3 (LSF), LaMnO3 (LSM), and LaCoO3 (LSCo). Density functional theory calculations were performed to determine the stability of different active sites on a collection of surfaces. With LSF and LSM, rates for the ORR are significantly higher on the A-site terminated surface, while surface termination is less important for LSCo. Our findings highlight the importance of tailoring the surface termination of the perovskite to obtain its ultimate ORR performance.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Jonathan Rodríguez-Fernández, Zhaozong Sun, Liang Zhang, Ting Tan, Anthony Curto, Jakob Fester, Aleksandra Vojvodic, Jeppe V Lauritsen, "Structural and electronic properties of Fe dopants in cobalt oxide nanoislands on Au(111)", The Journal of Chemical Physics, 150, 4, 2019, 041731.
@article{Rodriguez-Fernandez2019a,
title = {Structural and electronic properties of Fe dopants in cobalt oxide nanoislands on Au(111)},
author = {Jonathan Rodríguez-Fernández and Zhaozong Sun and Liang Zhang and Ting Tan and Anthony Curto and Jakob Fester and Aleksandra Vojvodic and Jeppe V Lauritsen},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/jcp1.5052336.pdf, PDF},
doi = {10.1063/1.5052336},
issn = {0021-9606},
year = {2019},
date = {2019-01-01},
journal = {The Journal of Chemical Physics},
volume = {150},
number = {4},
pages = {041731},
publisher = {American Institute of Physics Inc.},
abstract = {Mixed metal oxides of earth-abundant 3d transition metals are an interesting class of materials that show interesting magnetic properties and a significant synergistic effect as catalysts for electrochemical oxygen evolution compared to simple unary oxides. However, the exact atomic-scale nature of such mixed oxide phases and the link to their interesting physico-chemical properties are poorly understood. Here, a combination of scanning tunneling microscopy and x-ray photoemission spectroscopy reveals that Fe species embed in a facile way into CoO bilayers on Au(111) resulting in an Fe doped oxide. Density functional theory and the spectroscopic fingerprint from x-ray photoemission spectroscopy reveal that the Fe dopants in the cobalt oxide matrix assume a higher oxidation state than in the structurally corresponding unary bilayer oxide. Furthermore, the substituted Fe is structurally displaced further away from the Au than the metal in either of the corresponding pure unary oxides. Both O and to a smaller extent Co in the nearest coordination shell are also structurally and electronically perturbed. The interesting effects observed in the bilayer binary oxides may enable a better fundamental understanding of the nature of doping of metal oxides, in general, and promotion effects in catalytic applications.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

D Kwak, M J Wang, K J Koski, L Zhang, H Sokol, R Maric, Y Lei, "Molybdenum Trioxide (alpha-MoO3) Nanoribbons for Ultrasensitive Ammonia (NH3) Gas Detection: Integrated Experimental and Density Functional Theory Simulation Studies (vol 11, pg 10697, 2019)", Acs Applied Materials & Interfaces, 11, 40, 2019, 37379.
@article{Kwak2019,
title = {Molybdenum Trioxide (alpha-MoO3) Nanoribbons for Ultrasensitive Ammonia (NH3) Gas Detection: Integrated Experimental and Density Functional Theory Simulation Studies (vol 11, pg 10697, 2019)},
author = {D Kwak and M J Wang and K J Koski and L Zhang and H Sokol and R Maric and Y Lei},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acsami.8b20502.pdf, PDF},
doi = {10.1021/acsami.8b20502},
isbn = {1944-8244},
year = {2019},
date = {2019-01-01},
journal = {Acs Applied Materials & Interfaces},
volume = {11},
number = {40},
pages = {37379},
abstract = {A highly-sensitive ammonia (NH3) gas sensor based on molybdenum trioxide nanoribbons was developed in this study. α-MoO3 nanoribbons (MoO3 NRs) were successfully synthesized via a hydrothermal method and systematically characterized using various advanced technologies. Following a simple drop-cast process, a high-performance chemiresistive NH3 sensor was fabricated through the deposition of a MoO3 NR sensing film onto Au interdigitated electrodes. At an optimal operation temperature of 450 °C, the MoO3 nanoribbon-based sensor exhibited an excellent sensitivity (0.72) at NH3 concentration as low as 50 ppb, a fast response time of 21 s, good stability and reproducibility, and impressive selectivity against the interfering gases such as H2, NO2, and O2. More importantly, the sensor represents a remarkable limit of detection of 280 ppt (calculated based on a signal-to-noise ratio of 3), which makes the as-prepared MoO3 NR sensor the most sensitive NH3 sensor in the literature. Moreover, density functional theory (DFT) simulations were employed to understand the adsorption energetics and electronic structures and thus shed light on the fundamentals of sensing performance. The enhanced sensitivity for NH3 is explicitly discussed and explained by the remarkable band structure modification because of the NH3 adsorption at the oxygen vacancy site on α-MoO3 nanoribbons. These results verify that hydrothermally grown MoO3 nanoribbons are a promising sensing material for enhanced NH3 gas monitoring.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

2018
A R Akbashev, L Zhang, J T Mefford, J Park, B Butz, H Luftman, W C Chueh, A Vojvodic, "Activation of ultrathin SrTiO 3 with subsurface SrRuO 3 for the oxygen evolution reaction", Energy & Environmental Science, 11, 7, 2018, 1762-1769.
@article{Akbashev2018,
title = {Activation of ultrathin SrTiO 3 with subsurface SrRuO 3 for the oxygen evolution reaction},
author = {A R Akbashev and L Zhang and J T Mefford and J Park and B Butz and H Luftman and W C Chueh and A Vojvodic},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/c8ee00210j.pdf, PDF},
doi = {10.1039/C8EE00210J},
issn = {1754-5692},
year = {2018},
date = {2018-01-01},
urldate = {2018-01-01},
journal = {Energy & Environmental Science},
volume = {11},
number = {7},
pages = {1762--1769},
abstract = {Water electrolysis occurs via the oxygen/hydrogen evolution reactions. Achieving sufficiently high oxygen evolution reaction (OER) activity while maintaining stability of the active catalyst surface is a primary challenge of designing OER catalysts. Often high electrocatalytic activity is accompanied by structural instability. This is the case for the metallic perovskite oxide SrRuO3 (SRO), which exhibits rapid dissolution under OER conditions, both as thin-films and as nanoparticles. On the other hand, large band-gap perovskite oxides such as SrTiO3 (STO) are inactive for OER in the dark but are stable. In this work, we demonstrate that burying as little as one unit cell of SRO beneath an ultrathin STO capping layer activates the otherwise inert electrocatalyst and gives excellent stability. Using density functional theory (DFT) calculations, we find that such a chemical modification of STO by sub-surface SRO introduces new 4d electronic states including Ru states within the STO band gap, raising the energy level of the electrons, changing the electronic hybridization, and facilitating an easy transfer to the adsorbed intermediates. We validate this hypothesis experimentally using atomically precise heteroepitaxial deposition. We find that a single-unit-cell layer of SRO is sufficient to activate the topmost STO layer towards OER; burying SRO underneath two unit cells of STO protects the inherently unstable SRO against corrosion during OER. Generally, our layered heterostructures are model systems of oxide core–shell structures, where an unstable catalyst (core) is protected against degradation and electronically activates the otherwise inactive shell. As demonstrated in this work, the growth of ultrathin heterostructures establishes a rigorous platform for screening core/shell nanoparticle combinations/thicknesses, and elucidates sub-surface activation mechanism for achieving stable and active oxide electrocatalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Felicia R Lucci, Liang Zhang, Theodore Thuening, Matthew B Uhlman, Alex C Schilling, Graeme Henkelman, E Charles H. Sykes, "The effect of single pd atoms on the energetics of recombinative O2 desorption from Au(111)", Surface Science, 677, 2018, 296 - 300.
@article{LUCCI2018296,
title = {The effect of single pd atoms on the energetics of recombinative O2 desorption from Au(111)},
author = {Felicia R Lucci and Liang Zhang and Theodore Thuening and Matthew B Uhlman and Alex C Schilling and Graeme Henkelman and E Charles H. Sykes},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/1-s2.0-S003960281830517X-main.pdf, PDF},
doi = {https://doi.org/10.1016/j.susc.2018.08.001},
issn = {0039-6028},
year = {2018},
date = {2018-01-01},
journal = {Surface Science},
volume = {677},
pages = {296 - 300},
abstract = {The oxidation of gold is an important step in a number of catalytic reactions and the oxidation of Au(111) with ozone has been well-studied using surface science techniques. We report that the addition of 1% Pd in the surface in the form of a Pd/Au(111) single-atom alloy dramatically alters the desorption temperature of molecular oxygen after oxidation by ozone. We use temperature programmed desorption to study the desorption kinetics and scanning tunneling microscopy to compare the structure of the oxides produced on Au(111) with and without 1% Pd. Aided by density functional theory we hypothesize that the lower temperature evolution of O2 occurs not because Pd atoms lower the O2 desorption barrier, rather than the 1% Pd disrupts the formation of the more stable 2D ordered oxide formed on Au(11). This effect is particularly pronounced when the single-atom alloy surface is treated with ozone at lower temperatures.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

2017
Kihyun Shin, Liang Zhang, Hyesung An, Hyunwoo Ha, Mi Yoo, Hyuck Mo Lee, Graeme Henkelman, Hyun You Kim, "Interface engineering for a rational design of poison-free bimetallic CO oxidation catalysts", Nanoscale, 9, 16, 2017, 5244-5253.
@article{Shin2017,
title = {Interface engineering for a rational design of poison-free bimetallic CO oxidation catalysts},
author = {Kihyun Shin and Liang Zhang and Hyesung An and Hyunwoo Ha and Mi Yoo and Hyuck Mo Lee and Graeme Henkelman and Hyun You Kim},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/c7nr01382e.pdf, PDF},
doi = {10.1039/C7NR01382E},
issn = {2040-3364},
year = {2017},
date = {2017-01-01},
journal = {Nanoscale},
volume = {9},
number = {16},
pages = {5244--5253},
abstract = {We use density functional theory calculations of Pt@Cu core@shell nanoparticles (NPs) to design bifunctional poison-free CO oxidation catalysts. By calculating the adsorption chemistry under CO oxidation conditions, we find that the Pt@Cu NPs will be active for CO oxidation with resistance to CO-poisoning. The CO oxidation pathway at the Pt-Cu interface is determined on the Pt NP covered with a full- and partial-shell of Cu. The exposed portion of the Pt core preferentially binds CO and the Cu shell binds O2, supplying oxygen for the reaction. The Pt-Cu interface provides CO-oxidation sites that are not poisoned by either CO or O2. Additional computational screening shows that this separation of reactant binding sites is possible for several other core@shell NPs. Our results indicate that the metal-metal interface within a single NP can be optimized for design of bifunctional catalytic systems with improved performance.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2016
Benjamin Corona, Marco Howard, Liang Zhang, Graeme Henkelman, "Computational screening of core@shell nanoparticles for the hydrogen evolution and oxygen reduction reactions", The Journal of Chemical Physics, 145, 24, 2016, 244708.
@article{Corona2016,
title = {Computational screening of core@shell nanoparticles for the hydrogen evolution and oxygen reduction reactions},
author = {Benjamin Corona and Marco Howard and Liang Zhang and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/jcp1.4972579.pdf, PDF},
doi = {10.1063/1.4972579},
issn = {0021-9606},
year = {2016},
date = {2016-12-01},
journal = {The Journal of Chemical Physics},
volume = {145},
number = {24},
pages = {244708},
publisher = {American Institute of Physics Inc.},
abstract = {Using density functional theory calculations, a set of candidate nanoparticle catalysts are identified based on reactivity descriptors and segregation energies for the oxygen reduction and hydrogen evolution reactions. Trends in the data were identified by screening over 700 core@shell 2 nm transition metal nanoparticles for each reaction. High activity was found for nanoparticles with noble metal shells and a variety of core metals for both reactions. By screening for activity and stability, we obtain a set of interesting bimetallic catalysts, including cases that have reduced noble metal loadings and a higher predicted activity as compared to monometallic Pt nanoparticles.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Pranaw Kunal, Hao Li, Beth L Dewing, Liang Zhang, Karalee Jarvis, Graeme Henkelman, Simon M Humphrey, "Microwave-Assisted Synthesis of Pd x Au 100– x Alloy Nanoparticles: A Combined Experimental and Theoretical Assessment of Synthetic and Compositional Effects upon Catalytic Reactivity", ACS Catalysis, 6, 8, 2016, 4882-4893.
@article{Kunal2016,
title = {Microwave-Assisted Synthesis of Pd x Au 100– x Alloy Nanoparticles: A Combined Experimental and Theoretical Assessment of Synthetic and Compositional Effects upon Catalytic Reactivity},
author = {Pranaw Kunal and Hao Li and Beth L Dewing and Liang Zhang and Karalee Jarvis and Graeme Henkelman and Simon M Humphrey},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acscatal.6b01014.pdf, PDF},
doi = {10.1021/acscatal.6b01014},
issn = {2155-5435},
year = {2016},
date = {2016-08-01},
journal = {ACS Catalysis},
volume = {6},
number = {8},
pages = {4882--4893},
publisher = {American Chemical Society},
abstract = {PdxAu100–x nanoparticle (NP) catalysts with well-defined morphologies and compositions can be rapidly prepared using a simple microwave-assisted synthetic approach. Common Pd(II) and Au(III) precursors are coreduced in ethylene glycol to give small and nearly monodisperse (2.5 ± 0.6 nm) NPs with homogeneously alloyed structures in less than 300 s at 150 °C. A comparison of the nucleation and growth processes responsible for the formation of PdAuNPs by microwave and conventional methods revealed faster and more reproducible product formation under microwave-assisted heating. Pd-rich NPs were rapidly formed, into which Au atoms were subsequently incorporated to give the alloyed NPs. The value of x in the PdxAu100–xNPs obtained can be finely controlled, allowing the surface electronic structure of the NPs to be broadly tuned. This permits model heterogeneous reaction studies, in which catalytic reactivity can be directly related to Pd:Au composition. Vapor-phase alkene hydrogenation studies using a series of PdA},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Long Luo, Liang Zhang, Zhiyao Duan, Aliya S Lapp, Graeme Henkelman, Richard M Crooks, "Efficient CO Oxidation Using Dendrimer-Encapsulated Pt Nanoparticles Activated with< 2% Cu Surface Atoms", ACS nano, 10, 9, 2016, 8760-8769.
@article{luo2016efficient,
title = {Efficient CO Oxidation Using Dendrimer-Encapsulated Pt Nanoparticles Activated with< 2% Cu Surface Atoms},
author = {Long Luo and Liang Zhang and Zhiyao Duan and Aliya S Lapp and Graeme Henkelman and Richard M Crooks},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acsnano.6b04448.pdf, PDF},
doi = {10.1021/acsnano.6b04448},
year = {2016},
date = {2016-01-01},
journal = {ACS nano},
volume = {10},
number = {9},
pages = {8760--8769},
publisher = {ACS Publications},
abstract = {In this paper, we show that the onset potential for CO oxidation electrocatalyzed by ∼2 nm dendrimer-encapsulated Pt nanoparticles (Pt DENs) is shifted negative by ∼300 mV in the presence of a small percentage (<2%) of Cu surface atoms. Theory and experiments suggest that the catalytic enhancement arises from a cocatalytic Langmuir–Hinshelwood mechanism in which the small number of Cu atoms selectively adsorb OH, thereby facilitating reaction with CO adsorbed to the dominant Pt surface. Theory suggests that these Cu atoms are present primarily on the (100) facets of the Pt DENs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

2015
Penghao Xiao, Juliana Duncan, Liang Zhang, Graeme Henkelman, "Ridge-based bias potentials to accelerate molecular dynamics", The Journal of Chemical Physics, 143, 24, 2015, 244104.
@article{Xiao2015a,
title = {Ridge-based bias potentials to accelerate molecular dynamics},
author = {Penghao Xiao and Juliana Duncan and Liang Zhang and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/jcp1.4937393.pdf, PDF},
doi = {10.1063/1.4937393},
issn = {0021-9606},
year = {2015},
date = {2015-12-01},
journal = {The Journal of Chemical Physics},
volume = {143},
number = {24},
pages = {244104},
abstract = {An effective way to accelerate rare events in molecular dynamics simulations is to apply a bias potential which destabilizes minima without biasing the transitions between stable states. This approach, called hyperdynamics, is limited by our ability to construct general bias potentials without having to understand the reaction mechanisms available to the system, a priori. Current bias potentials are typically constructed in terms of a metric which quantifies the distance that a trajectory deviates from the reactant state minimum. Such metrics include detection of negative curvatures of the potential, an energy increase, or deviations in bond lengths from the minimum. When one of these properties exceeds a critical value, the bias potentials are constructed to approach zero. A problem common to each of these schemes is that their effectiveness decreases rapidly with system size. We attribute this problem to a diminishing volume defined by the metrics around a reactant minimum as compared to the total volume of the reactant state basin. In this work, we mitigate the dimensionality scaling problem by constructing bias potentials that are based upon the distance to the boundary of the reactant basin. This distance is quantified in two ways: (i) by following the minimum mode direction to the reactant boundary and (ii) by training a machine learning algorithm to give an analytic expression for the boundary to which the distance can be calculated. Both of these ridge-based bias potentials are demonstrated to scale qualitatively better with dimensionality than the existing methods. We attribute this improvement to a greater filling fraction of the reactant state using the ridge-based bias potentials as compared to the standard potentials.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Rachel M Anderson, Liang Zhang, Dongjun Wu, Stanko R Brankovic, Graeme Henkelman, Richard M Crooks, "A Theoretical and Experimental In-Situ Electrochemical Infrared Spectroscopy Study of Adsorbed CO on Pt Dendrimer-Encapsulated Nanoparticles", Journal of The Electrochemical Society, 163, 4, 2015, H3061-H3065.
@article{Anderson_2015,
title = {A Theoretical and Experimental In-Situ Electrochemical Infrared Spectroscopy Study of Adsorbed CO on Pt Dendrimer-Encapsulated Nanoparticles},
author = {Rachel M Anderson and Liang Zhang and Dongjun Wu and Stanko R Brankovic and Graeme Henkelman and Richard M Crooks},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/Anderson_2016_J._Electrochem._Soc._163_H3061.pdf, PDF},
doi = {10.1149/2.0061604jes},
year = {2015},
date = {2015-12-01},
journal = {Journal of The Electrochemical Society},
volume = {163},
number = {4},
pages = {H3061--H3065},
publisher = {The Electrochemical Society},
abstract = {We present a combined experimental and theoretical study of COads on Pt147 dendrimer-encapsulated nanoparticles (DENs). In-situ electrochemical IR spectroscopy reveals an 8 cm−1 redshift of the COads stretching frequency on Pt147 DENs relative to a Pt(111) crystal. This value is in good agreement with the shift calculated by density functional theory. Importantly, the wavenumber shift observed in this study is significantly smaller than has been found previously. We attribute this primarily to the absence of support effects and the narrow size distribution of DENs. The agreement between experiment and theory validates the model nanoparticle system used for the calculations, and this will make it possible to use the COads frequency as a probe to study more complex DEN structures and as a descriptor of the catalytic activity of DENs toward reactions such as formic acid oxidation and methanol oxidation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Liang Zhang, Samuel T Chill, Graeme Henkelman, "Distributed replica dynamics", The Journal of Chemical Physics, 143, 17, 2015, 174112.
@article{Zhang2015c,
title = {Distributed replica dynamics},
author = {Liang Zhang and Samuel T Chill and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/JCP1.4934987.pdf, PDF},
doi = {10.1063/1.4934987},
issn = {0021-9606},
year = {2015},
date = {2015-11-01},
urldate = {2015-11-01},
journal = {The Journal of Chemical Physics},
volume = {143},
number = {17},
pages = {174112},
abstract = {A distributed replica dynamics (DRD) method is proposed to calculate rare-event molecular dynamics using distributed computational resources. Similar to Voter's parallel replica dynamics (PRD) method, the dynamics of independent replicas of the system are calculated on different computational clients. In DRD, each replica runs molecular dynamics from an initial state for a fixed simulation time and then reports information about the trajectory back to the server. A simulation clock on the server accumulates the simulation time of each replica until one reports a transition to a new state. Subsequent calculations are initiated from within this new state and the process is repeated to follow the state-to-state evolution of the system. DRD is designed to work with asynchronous and distributed computing resources in which the clients may not be able to communicate with each other. Additionally, clients can be added or removed from the simulation at any point in the calculation. Even with heterogeneous computing clients, we prove that the DRD method reproduces the correct probability distribution of escape times. We also show this correspondence numerically; molecular dynamics simulations of Al(100) adatom diffusion using PRD and DRD give consistent exponential distributions of escape times. Finally, we discuss guidelines for choosing the optimal number of replicas and replica trajectory length for the DRD method.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Liang Zhang, Rachel M Anderson, Richard M Crooks, Graeme Henkelman, "Correlating Structure and Function of Metal Nanoparticles for Catalysis", Surface Science, 640, 2015, 65-72.
@article{Zhang2015a,
title = {Correlating Structure and Function of Metal Nanoparticles for Catalysis},
author = {Liang Zhang and Rachel M Anderson and Richard M Crooks and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/1-s2.0-S0039602815000783-main.pdf, PDF},
doi = {10.1016/j.susc.2015.03.018},
issn = {00396028},
year = {2015},
date = {2015-10-01},
journal = {Surface Science},
volume = {640},
pages = {65--72},
abstract = {This paper summarizes several studies correlating the structure and function of nanoparticle catalysts. Three types of alloy nanoparticles are considered, random alloy, core@shell and alloy-core@shell structures. In the first two cases, the focus is to build theoretical models to understand previous experimental results. In the latter case, calculations play a greater role in leading the development of nanoparticle catalysts. We demonstrate that iteration between theory and experiment can facilitate an understanding of nanoparticle catalysts and reduce the time and effort involved in the design of new catalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Long Luo, Liang Zhang, Graeme Henkelman, Richard M Crooks, "Unusual Activity Trend for CO Oxidation on Pd x Au 140– x @Pt Core@Shell Nanoparticle Electrocatalysts", The Journal of Physical Chemistry Letters, 6, 13, 2015, 2562-2568.
@article{Luo2015,
title = {Unusual Activity Trend for CO Oxidation on Pd x Au 140– x @Pt Core@Shell Nanoparticle Electrocatalysts},
author = {Long Luo and Liang Zhang and Graeme Henkelman and Richard M Crooks},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.jpclett.5b00985.pdf, PDF},
doi = {10.1021/acs.jpclett.5b00985},
issn = {1948-7185},
year = {2015},
date = {2015-07-01},
journal = {The Journal of Physical Chemistry Letters},
volume = {6},
number = {13},
pages = {2562--2568},
abstract = {A theoretical and experimental study of the electrocatalytic oxidation of CO on PdxAu140-x@Pt dendrimer-encapsulated nanoparticle (DEN) catalysts is presented. These nanoparticles are comprised of a core having an average of 140 atoms and a Pt monolayer shell. The CO oxidation activity trend exhibits an unusual koppa shape as the number of Pd atoms in the core is varied from 0 to 140. Calculations based on density functional theory suggest that the koppa-shaped trend is driven primarily by structural changes that affect the CO binding energy on the surface. Specifically, a pure Au core leads to deformation of the Pt shell and a compression of the Pt lattice. In contrast, Pd, from the pure Pd cores, tends to segregate on the DEN surface, forming an inverted configuration having Pt within the core and Pd in the shell. With a small addition of Au, however, the alloy PdAu cores stabilize the core@shell structures by preventing Au and Pd from escaping to the particle surface.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Rachel M Anderson, David F Yancey, Liang Zhang, Samuel T Chill, Graeme Henkelman, Richard M Crooks, "A Theoretical and Experimental Approach for Correlating Nanoparticle Structure and Electrocatalytic Activity", Accounts of Chemical Research, 48, 5, 2015, 1351-1357.
@article{Anderson2015,
title = {A Theoretical and Experimental Approach for Correlating Nanoparticle Structure and Electrocatalytic Activity},
author = {Rachel M Anderson and David F Yancey and Liang Zhang and Samuel T Chill and Graeme Henkelman and Richard M Crooks},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.accounts.5b00125.pdf, PDF},
doi = {10.1021/acs.accounts.5b00125},
issn = {0001-4842},
year = {2015},
date = {2015-05-01},
journal = {Accounts of Chemical Research},
volume = {48},
number = {5},
pages = {1351--1357},
abstract = {ConspectusThe objective of the research described in this Account is the development of high-throughput computational-based screening methods for discovery of catalyst candidates and subsequent experimental validation using appropriate catalytic nanoparticles. Dendrimer-encapsulated nanoparticles (DENs), which are well-defined 1-2 nm diameter metal nanoparticles, fulfill the role of model electrocatalysts.Effective comparison of theory and experiment requires that the theoretical and experimental models map onto one another perfectly. We use novel synthetic methods, advanced characterization techniques, and density functional theory (DFT) calculations to approach this ideal. For example, well-defined core@shell DENs can be synthesized by electrochemical underpotential deposition (UPD), and the observed deposition potentials can be compared to those calculated by DFT. Theory is also used to learn more about structure than can be determined by analytical characterization alone. For example, density functional theory molecular dynamics (DFT-MD) was used to show that the core@shell configuration of Au@Pt DENs undergoes a surface reconstruction that dramatically affects its electrocatalytic properties. A separate Pd@Pt DENs study also revealed reorganization, in this case a core-shell inversion to a Pt@Pd structure. Understanding these types of structural changes is critical to building correlations between structure and catalytic function.Indeed, the second principal focus of the work described here is correlating structure and catalytic function through the combined use of theory and experiment. For example, the Au@Pt DENs system described earlier is used for the oxygen reduction reaction (ORR) as well as for the electro-oxidation of formic acid. The surface reorganization predicted by theory enhances our understanding of the catalytic measurements. In the case of formic acid oxidation, the deformed nanoparticle structure leads to reduced CO binding energy and therefore improved oxidation activity. The final catalytic study we present is an instance of theory correctly predicting (in advance of the experiments) the structure of an effective DEN electrocatalyst. Specifically, DFT was used to determine the optimal composition of the alloy-core in AuPd@Pt DENs for the ORR. This prediction was subsequently confirmed experimentally. This study highlights the major theme of our research: the progression of using theory to rationalize experimental results to the more advanced goal of using theory to predict catalyst function a priori. We still have a long way to go before theory will be the principal means of catalyst discovery, but this Account begins to shed some light on the path that may lead in that direction.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Wen-Yueh Yu, Liang Zhang, Gregory M Mullen, Graeme Henkelman, Buddie C Mullins, "Oxygen Activation and Reaction on Pd–Au Bimetallic Surfaces", The Journal of Physical Chemistry C, 119, 21, 2015, 11754-11762.
@article{Yu2015,
title = {Oxygen Activation and Reaction on Pd–Au Bimetallic Surfaces},
author = {Wen-Yueh Yu and Liang Zhang and Gregory M Mullen and Graeme Henkelman and Buddie C Mullins},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/acs.jpcc.5b02970.pdf, PDF},
doi = {10.1021/acs.jpcc.5b02970},
issn = {1932-7447},
year = {2015},
date = {2015-05-01},
journal = {The Journal of Physical Chemistry C},
volume = {119},
number = {21},
pages = {11754--11762},
abstract = {We report electrocatalytic oxidation of formic acid using monometallic and bimetallic dendrimer-encapsulated nanoparticles (DENs). The results indicate that the Au147@Pt DENs exhibit better electrocatalytic activity and low CO formation. Theoretical calculations attribute the observed activity to the deformation of nanoparticle structure, slow dehydration of formic acid, and weak binding of CO on Au147@Pt surface. Subsequent experiments confirmed the theoretical predictions. textcopyright 2013 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Liang Zhang, Graeme Henkelman, "Computational Design of Alloy-Core@Shell Metal Nanoparticle Catalysts", ACS Catalysis, 5, 2, 2015, 655-660.
@article{Zhang2015b,
title = {Computational Design of Alloy-Core@Shell Metal Nanoparticle Catalysts},
author = {Liang Zhang and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/cs501176b.pdf, PDF},
doi = {10.1021/cs501176b},
issn = {2155-5435},
year = {2015},
date = {2015-02-01},
urldate = {2015-02-01},
journal = {ACS Catalysis},
volume = {5},
number = {2},
pages = {655--660},
abstract = {The alloy-core@shell nanoparticle structure combines the advantages of a robust noble-metal shell and a tunable alloy-core composition. In this study we demonstrate a set of linear correlations between the binding of adsorbates to the shell and the alloy-core composition, which are general across a range of nanoparticle compositions, size, and adsorbate molecules. This systematic tunability allows for a simple approach to the design of such catalysts. Calculations of candidate structures for the hydrogen evolution reaction predict a high activity for the PtRu@Pd structure, in good agreement with what has been reported previously. Calculations of alloy-core@Pt 140-atom nanoparticles reveal new candidate structures for CO oxidation at high temperature, including Au0.65Pd0.35@Pt and Au0.73Pt0.27@Pt, which are predicted to have reaction rates 200 times higher than that of Pt(111).},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Wen-Yueh Yu, Liang Zhang, Gregory M Mullen, Edward J Evans, Graeme Henkelman, Buddie C Mullins, "Effect of annealing in oxygen on alloy structures of Pd–Au bimetallic model catalysts", Physical Chemistry Chemical Physics, 17, 32, 2015, 20588-20596.
@article{Yu2015a,
title = {Effect of annealing in oxygen on alloy structures of Pd–Au bimetallic model catalysts},
author = {Wen-Yueh Yu and Liang Zhang and Gregory M Mullen and Edward J Evans and Graeme Henkelman and Buddie C Mullins},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/c5cp03515e.pdf, PDF},
doi = {10.1039/C5CP03515E},
issn = {1463-9076},
year = {2015},
date = {2015-01-01},
journal = {Physical Chemistry Chemical Physics},
volume = {17},
number = {32},
pages = {20588--20596},
abstract = {It has been reported that Pd–Au bimetallic catalysts display improved catalytic performance after adequate calcination. In this study, a model catalyst study was conducted to investigate the effects of annealing in oxygen on the surface structures of Pd–Au alloys by comparing the physicochemical properties of Pd/Au(111) surfaces that were annealed in ultrahigh vacuum (UHV) versus in an oxygen ambient. Auger electron spectroscopy (AES) and Basin hopping simulations reveal that the presence of oxygen can inhibit the diffusion of surface Pd atoms into the subsurface of the Au(111) sample. Reflection–absorption infrared spectroscopy using CO as a probe molecule (CO-RAIRS) and King–Wells measurements of O2 uptake suggest that surfaces annealed in an oxygen ambient possess more contiguous Pd sites than surfaces annealed under UHV conditions. The oxygen-annealed Pd/Au(111) surface exhibited a higher activity for CO oxidation in reactive molecular beam scattering (RMBS) experiments. This enhanced activity likely results from the higher oxygen uptake and relatively facile dissociation of oxygen admolecules due to stronger adsorbate–surface interactions as suggested by temperature-programmed desorption (TPD) measurements. These observations provide fundamental insights into the surface phenomena of Pd–Au alloys, which may prove beneficial in the design of future Pd–Au catalysts.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Gregory M Mullen, Liang Zhang, Edward J Evans, Ting Yan, Graeme Henkelman, Buddie C Mullins, "Control of selectivity in allylic alcohol oxidation on gold surfaces: the role of oxygen adatoms and hydroxyl species", Physical Chemistry Chemical Physics, 17, 6, 2015, 4730-4738.
@article{Mullen2015,
title = {Control of selectivity in allylic alcohol oxidation on gold surfaces: the role of oxygen adatoms and hydroxyl species},
author = {Gregory M Mullen and Liang Zhang and Edward J Evans and Ting Yan and Graeme Henkelman and Buddie C Mullins},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/c4cp04739g.pdf, PDF},
doi = {10.1039/C4CP04739G},
issn = {1463-9076},
year = {2015},
date = {2015-01-01},
journal = {Physical Chemistry Chemical Physics},
volume = {17},
number = {6},
pages = {4730--4738},
abstract = {Gold catalysts display high activity and good selectivity for partial oxidation of a number of alcohol species. In this work, we discuss the effects of oxygen adatoms and surface hydroxyls on the selectivity for oxidation of allylic alcohols (allyl alcohol and crotyl alcohol) on gold surfaces. Utilizing temperature programmed desorption (TPD), reactive molecular beam scattering (RMBS), and density functional theory (DFT) techniques, we provide evidence to suggest that the selectivity displayed towards partial oxidation versus combustion pathways is dependent on the type of oxidant species present on the gold surface. TPD and RMBS results suggest that surface hydroxyls promote partial oxidation of allylic alcohols to their corresponding aldehydes with very high selectivity, while oxygen adatoms promote both partial oxidation and combustion pathways. DFT calculations indicate that oxygen adatoms can react with acrolein to promote the formation of a bidentate surface intermediate, similar to structures that have been shown to decompose to generate combustion products over other transition metal surfaces. Surface hydroxyls do not readily promote such a process. Our results help explain phenomena observed in previous studies and may prove useful in the design of future catalysts for partial oxidation of alcohols.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2014
Stephany García, Liang Zhang, Graham W Piburn, Graeme Henkelman, Simon M Humphrey, "Microwave Synthesis of Classically Immiscible Rhodium–Silver and Rhodium–Gold Alloy Nanoparticles: Highly Active Hydrogenation Catalysts", ACS Nano, 8, 11, 2014, 11512-11521.
@article{Garcia2014,
title = {Microwave Synthesis of Classically Immiscible Rhodium–Silver and Rhodium–Gold Alloy Nanoparticles: Highly Active Hydrogenation Catalysts},
author = {Stephany García and Liang Zhang and Graham W Piburn and Graeme Henkelman and Simon M Humphrey},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/nn504746u.pdf, PDF},
doi = {10.1021/nn504746u},
issn = {1936-0851},
year = {2014},
date = {2014-11-01},
journal = {ACS Nano},
volume = {8},
number = {11},
pages = {11512--11521},
abstract = {Noble metal alloys are important in large-scale catalytic processes. Alloying facilitates fine-tuning of catalytic properties via synergistic interactions between metals. It also allows for dilution of scarce and expensive metals using comparatively earth-abundant metals. RhAg and RhAu are classically considered to be immiscible metals. We show here that stable RhM (M = Ag, Au) nanoparticles with randomly alloyed structures and broadly tunable Rh:M ratios can be prepared using a microwave-assisted method. The alloyed nanostructures with optimized Rh:M compositions are significantly more active as hydrogenation catalysts than Rh itself: Rh is more dilute and more reactive when alloyed with Ag or Au, even though the latter are both catalytically inactive for hydrogenation. Theoretical modeling predicts that the observed catalytic enhancement is due to few-atom surface ensemble effects in which the overall reaction energy profile for alkene hydrogenation is optimized due to RhM d-band intermixing.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Samuel T Chill, Matthew Welborn, Rye Terrell, Liang Zhang, Jean-Claude Berthet, Andreas Pedersen, Hannes Jónsson, Graeme Henkelman, "EON: software for long time simulations of atomic scale systems", Modelling and Simulation in Materials Science and Engineering, 22, 5, 2014, 055002.
@article{Chill2014,
title = {EON: software for long time simulations of atomic scale systems},
author = {Samuel T Chill and Matthew Welborn and Rye Terrell and Liang Zhang and Jean-Claude Berthet and Andreas Pedersen and Hannes Jónsson and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/Chill_2014_Modelling_Simul._Mater._Sci._Eng._22_055002.pdf, PDF},
doi = {10.1088/0965-0393/22/5/055002},
issn = {0965-0393},
year = {2014},
date = {2014-07-01},
journal = {Modelling and Simulation in Materials Science and Engineering},
volume = {22},
number = {5},
pages = {055002},
abstract = {The EON software is designed for simulations of the state-to-state evolution of atomic scale systems over timescales greatly exceeding that of direct classical dynamics. States are defined as collections of atomic configurations from which a minimization of the potential energy gives the same inherent structure. The time evolution is assumed to be governed by rare events, where transitions between states are uncorrelated and infrequent compared with the timescale of atomic vibrations. Several methods for calculating the state-to-state evolution have been implemented in EON, including parallel replica dynamics, hyperdynamics and adaptive kinetic Monte Carlo. Global optimization methods, including simulated annealing, basin hopping and minima hopping are also implemented. The software has a client/server architecture where the computationally intensive evaluations of the interatomic interactions are calculated on the client-side and the state-to-state evolution is managed by the server. The client supports optimization for different computer architectures to maximize computational efficiency. The server is written in Python so that developers have access to the high-level functionality without delving into the computationally intensive components. Communication between the server and clients is abstracted so that calculations can be deployed on a single machine, clusters using a queuing system, large parallel computers using a message passing interface, or within a distributed computing environment. A generic interface to the evaluation of the interatomic interactions is defined so that empirical potentials, such as in LAMMPS, and density functional theory as implemented in VASP and GPAW can be used interchangeably. Examples are given to demonstrate the range of systems that can be modeled, including surface diffusion and island ripening of adsorbed atoms on metal surfaces, molecular diffusion on the surface of ice and global structural optimization of nanoparticles. textcopyright 2014 IOP Publishing Ltd.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
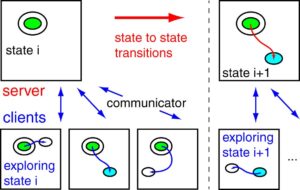
Gregory M Mullen, Liang Zhang, Edward J Evans, Ting Yan, Graeme Henkelman, Buddie C Mullins, "Oxygen and Hydroxyl Species Induce Multiple Reaction Pathways for the Partial Oxidation of Allyl Alcohol on Gold", Journal of the American Chemical Society, 136, 17, 2014, 6489-6498.
@article{Mullen2014,
title = {Oxygen and Hydroxyl Species Induce Multiple Reaction Pathways for the Partial Oxidation of Allyl Alcohol on Gold},
author = {Gregory M Mullen and Liang Zhang and Edward J Evans and Ting Yan and Graeme Henkelman and Buddie C Mullins},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/ja502347d.pdf, PDF},
doi = {10.1021/ja502347d},
issn = {0002-7863},
year = {2014},
date = {2014-04-01},
journal = {Journal of the American Chemical Society},
volume = {136},
number = {17},
pages = {6489--6498},
abstract = {Partial oxidation of alcohols is a topic of great interest in the field of gold catalysis. In this work, we provide evidence that the partial oxidation of allyl alcohol to its corresponding aldehyde, acrolein, over oxygen-precovered gold surfaces occurs via multiple reaction pathways. Utilizing temperature-programmed desorption (TPD) with isotopically labeled water and oxygen species, reactive molecular beam scattering, and density functional theory (DFT) calculations, we demonstrate that the reaction mechanism for allyl alcohol oxidation is influenced by the relative proportions of atomic oxygen and hydroxyl species on the gold surface. Both atomic oxygen and hydroxyl species are shown to be active for allyl alcohol oxidation, but each displays a different pathway of oxidation, as indicated by TPD measurements and DFT calculations. The hydroxyl hydrogen of allyl alcohol is readily abstracted by either oxygen adatoms or adsorbed hydroxyl species on the gold surface to generate a surface-bound allyloxide intermediate, which then undergoes $alpha$-dehydrogenation via interaction with an oxygen adatom or surface hydroxyl species to generate acrolein. Mediation of a second allyloxide with the hydroxyl species lowers the activation barrier for the $alpha$-dehydrogenation process. A third pathway exists in which two hydroxyl species recombine to generate water and an oxygen adatom, which subsequently dehydrogenates allyloxide. This work may aid in the understanding of oxidative catalysis over gold and the effect of water therein. textcopyright 2014 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

2013
Liang Zhang, Ravikumar Iyyamperumal, David F Yancey, Richard M Crooks, Graeme Henkelman, "Design of Pt-Shell Nanoparticles with Alloy Cores for the Oxygen Reduction Reaction", ACS Nano, 7, 10, 2013, 9168-9172.
@article{Zhang2013a,
title = {Design of Pt-Shell Nanoparticles with Alloy Cores for the Oxygen Reduction Reaction},
author = {Liang Zhang and Ravikumar Iyyamperumal and David F Yancey and Richard M Crooks and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/nn403788a.pdf, PDF},
doi = {10.1021/nn403788a},
issn = {1936-0851},
year = {2013},
date = {2013-10-01},
journal = {ACS Nano},
volume = {7},
number = {10},
pages = {9168--9172},
abstract = {We report that the oxygen binding energy of alloy-core@Pt nanoparticles can be linearly tuned by varying the alloy-core composition. Using this tuning mechanism, we are able to predict optimal compositions for different alloy-core@Pt nanoparticles. Subsequent electrochemical measurements of ORR activities of AuPd@Pt dendrimer-encapsulated nanoparticles (DENs) are in a good agreement with the theoretical prediction that the peak of activity is achieved for a 28% Au/72% Pd alloy core supporting a Pt shell. Importantly, these findings represent an unusual case of first-principles theory leading to nearly perfect agreement with experimental results. textcopyright 2013 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Rachel M Anderson, Liang Zhang, James A Loussaert, Anatoly I Frenkel, Graeme Henkelman, Richard M Crooks, "An Experimental and Theoretical Investigation of the Inversion of Pd@Pt Core@Shell Dendrimer-Encapsulated Nanoparticles", ACS Nano, 7, 10, 2013, 9345-9353.
@article{Anderson2013,
title = {An Experimental and Theoretical Investigation of the Inversion of Pd@Pt Core@Shell Dendrimer-Encapsulated Nanoparticles},
author = {Rachel M Anderson and Liang Zhang and James A Loussaert and Anatoly I Frenkel and Graeme Henkelman and Richard M Crooks},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/nn4040348.pdf, PDF},
doi = {10.1021/nn4040348},
issn = {1936-0851},
year = {2013},
date = {2013-10-01},
journal = {ACS Nano},
volume = {7},
number = {10},
pages = {9345--9353},
abstract = {Bimetallic PdPt dendrimer-encapsulated nanoparticles (DENs) having sizes of about 2 nm were synthesized by a homogeneous route that involved (1) formation of a Pd core, (2) deposition of a Cu shell onto the Pd core in the presence of H2 gas, and (3) galvanic exchange of Pt for the Cu shell. Under these conditions, a Pd@Pt core@shell DEN is anticipated, but detailed characterization by in-situ extended X-ray absorption fine structure (EXAFS) spectroscopy and other analytical methods indicate that the metals invert to yield a Pt-rich core with primarily Pd in the shell. The experimental findings correlate well with density functional theoretical (DFT) calculations. Theory suggests that the increased disorder associated with textless∼2 nm diameter nanoparticles, along with the relatively large number of edge and corner sites, drives the structural rearrangement. This type of rearrangement is not observed on larger nanoparticles or in bulk metals. textcopyright 2013 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Liang Zhang, Hyun You Kim, Graeme Henkelman, "CO Oxidation at the Au–Cu Interface of Bimetallic Nanoclusters Supported on CeO 2 (111)", The Journal of Physical Chemistry Letters, 4, 17, 2013, 2943-2947.
@article{Zhang2013b,
title = {CO Oxidation at the Au–Cu Interface of Bimetallic Nanoclusters Supported on CeO 2 (111)},
author = {Liang Zhang and Hyun You Kim and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/jz401524d.pdf, PDF},
doi = {10.1021/jz401524d},
issn = {1948-7185},
year = {2013},
date = {2013-09-01},
journal = {The Journal of Physical Chemistry Letters},
volume = {4},
number = {17},
pages = {2943--2947},
abstract = {DFT+U calculations of the structure of CeO2(111)-supported Au-based bimetallic nanoclusters (NCs) show that a strong support-metal interaction induces a preferential segregation of the more reactive element to the NC-CeO2 perimeter, generating an interface with the Au component. We studied several Au -based bimetallic NCs (Au-X, X: Ag, Cu, Pd, Pt, Rh, and Ru) and found that (Au-Cu)/CeO2 is optimal for catalyzing CO oxidation via a bifunctional mechanism. O2 preferentially binds to the Cu-rich sites, whereas CO binds to the Au-rich sites. Engineering a two-component system in which the reactants do not compete for binding sites is the key to the high catalytic activity at the interface between the components. textcopyright 2013 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Ravikumar Iyyamperumal, Liang Zhang, Graeme Henkelman, Richard M Crooks, "Efficient Electrocatalytic Oxidation of Formic Acid Using Au@Pt Dendrimer-Encapsulated Nanoparticles", Journal of the American Chemical Society, 135, 15, 2013, 5521-5524.
@article{Iyyamperumal2013,
title = {Efficient Electrocatalytic Oxidation of Formic Acid Using Au@Pt Dendrimer-Encapsulated Nanoparticles},
author = {Ravikumar Iyyamperumal and Liang Zhang and Graeme Henkelman and Richard M Crooks},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/ja4010305.pdf, PDF},
doi = {10.1021/ja4010305},
issn = {0002-7863},
year = {2013},
date = {2013-04-01},
journal = {Journal of the American Chemical Society},
volume = {135},
number = {15},
pages = {5521--5524},
publisher = {American Chemical Society},
abstract = {We report electrocatalytic oxidation of formic acid using monometallic and bimetallic dendrimer-encapsulated nanoparticles (DENs). The results indicate that the Au147@Pt DENs exhibit better electrocatalytic activity and low CO formation. Theoretical calculations attribute the observed activity to the deformation of nanoparticle structure, slow dehydration of formic acid, and weak binding of CO on Au147@Pt surface. Subsequent experiments confirmed the theoretical predictions. textcopyright 2013 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

David F Yancey, Samuel T Chill, Liang Zhang, Anatoly I Frenkel, Graeme Henkelman, Richard M Crooks, "A theoretical and experimental examination of systematic ligand-induced disorder in Au dendrimer-encapsulated nanoparticles", Chemical Science, 4, 7, 2013, 2912.
@article{Yancey2013,
title = {A theoretical and experimental examination of systematic ligand-induced disorder in Au dendrimer-encapsulated nanoparticles},
author = {David F Yancey and Samuel T Chill and Liang Zhang and Anatoly I Frenkel and Graeme Henkelman and Richard M Crooks},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/c3sc50614b.pdf, PDF},
doi = {10.1039/c3sc50614b},
issn = {2041-6520},
year = {2013},
date = {2013-01-01},
journal = {Chemical Science},
volume = {4},
number = {7},
pages = {2912},
abstract = {In this paper we present a new methodology for the analysis of 1-2 nm nanoparticles using extended X-ray absorption fine structure (EXAFS) spectroscopy. Different numbers of thiols were introduced onto the surfaces of dendrimer-encapsulated Au nanoparticles, consisting of an average of 147 atoms, to systematically tune the nanoparticle disorder. An analogous system was investigated using density functional theory molecular dynamics (DFT-MD) simulations to produce theoretical EXAFS signals that could be directly compared to the experimental results. Validation of the theoretical results by comparing to experiment allows us to infer previously unknown details of structure and dynamics of the nanoparticles. Additionally, the structural information that is learned from theoretical studies can be compared with traditional EXAFS fitting results to identify and rationalize any errors in the experimental fit. This study demonstrates that DFT-MD simulations accurately depict complex experimental systems in which we have control over nanoparticle disorder, and shows the advantages of using a combined experimental/theoretical approach over standard EXAFS fitting methodologies for determining the structural parameters of metallic nanoparticles. textcopyright 2013 Royal Society of Chemistry.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
2012
Liang Zhang, Graeme Henkelman, "Tuning the Oxygen Reduction Activity of Pd Shell Nanoparticles with Random Alloy Cores", The Journal of Physical Chemistry C, 116, 39, 2012, 20860-20865.
@article{Zhang2012,
title = {Tuning the Oxygen Reduction Activity of Pd Shell Nanoparticles with Random Alloy Cores},
author = {Liang Zhang and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/jp305367z.pdf, PDF},
doi = {10.1021/jp305367z},
issn = {1932-7447},
year = {2012},
date = {2012-10-01},
journal = {The Journal of Physical Chemistry C},
volume = {116},
number = {39},
pages = {20860--20865},
abstract = {Pd-based nanoparticles are promising candidates for non-Pt catalysts of the oxygen reduction reaction (ORR). Trends in ORR activity of Pd/Cu-alloy-core@Pd- shell nanoparticles are studied by calculating the oxygen binding energy on the Pd surface with different Cu compositions in the alloy core. Density functional theory calculations show that several properties of the nanoparticle surface, including the average oxygen binding energy, d-band center, and the net charge of Pd, are linearly related to the ratio of Cu in the core, demonstrating the capacity to tune ORR activity. Trends in oxygen binding of other core alloys are also studied and show similar linear trends with core composition, providing a design strategy for new ORR catalysts. textcopyright 2012 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

David F Yancey, Liang Zhang, Richard M Crooks, Graeme Henkelman, "Au@Pt dendrimer encapsulated nanoparticles as model electrocatalysts for comparison of experiment and theory", Chemical Science, 3, 4, 2012, 1033.
@article{Yancey2012,
title = {Au@Pt dendrimer encapsulated nanoparticles as model electrocatalysts for comparison of experiment and theory},
author = {David F Yancey and Liang Zhang and Richard M Crooks and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/c2sc00971d.pdf, PDF},
doi = {10.1039/c2sc00971d},
issn = {2041-6520},
year = {2012},
date = {2012-01-01},
journal = {Chemical Science},
volume = {3},
number = {4},
pages = {1033},
abstract = {In this paper we report the electrochemical synthesis of core@shell dendrimer-encapsulated nanoparticles (DENs) consisting of cores containing 147 Au atoms (Autextlessinftextgreater147textless/inftextgreater) and Pt shells having ∼54 or ∼102 atoms (Autextlessinftextgreater147textless/inftextgreater@Pttextlessinftextgreaterntextless/inftextgreater (n = 54 or 102)). The significance of this work arises from the correlation of the experimentally determined structural and electrocatalytic properties of these particles with density functional theory (DFT) calculations. Specifically, we describe an experimental and theoretical study of Pb underpotential deposition (UPD) on Autextlessinftextgreater147textless/inftextgreater DENs, the structure of both Autextlessinftextgreater147textless/inftextgreater@Pbtextlessinftextgreaterntextless/inftextgreater and Autextlessinftextgreater147textless/inftextgreater@Pt textlessinftextgreaterntextless/inftextgreater DENs, and the activity of these DENs for the oxygen reduction reaction (ORR). DFT calculations show that Pb binding is stronger on the (100) facets of Au as compared to (111), and the calculated deposition and stripping potentials are consistent with those measured experimentally. Galvanic exchange is used to replace the surface Pb atoms with Pt, and a surface distortion is found for Autextlessinftextgreater147textless/inftextgreater@Pttextlessinftextgreaterntextless/inftextgreater particles using molecular dynamics simulations in which the Pt-covered (100) facets shear into (111) diamond structures. DFT calculations of oxygen binding show that the distorted surfaces are the most active for the ORR, and that their activity is similar regardless of the Pt coverage. These calculations are consistent with rotating ring-disk voltammetry measurements. textcopyright 2012 The Royal Society of Chemistry.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
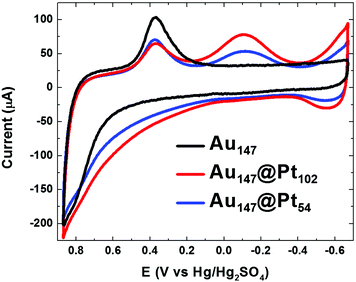
2011
Wenjie Tang, Liang Zhang, Graeme Henkelman, "Catalytic Activity of Pd/Cu Random Alloy Nanoparticles for Oxygen Reduction", The Journal of Physical Chemistry Letters, 2, 11, 2011, 1328-1331.
@article{Tang2011,
title = {Catalytic Activity of Pd/Cu Random Alloy Nanoparticles for Oxygen Reduction},
author = {Wenjie Tang and Liang Zhang and Graeme Henkelman},
url = {https://zhanglab-thu.com/wp-content/uploads/2020/12/publications/jz2004717.pdf, PDF},
doi = {10.1021/jz2004717},
issn = {1948-7185},
year = {2011},
date = {2011-06-01},
journal = {The Journal of Physical Chemistry Letters},
volume = {2},
number = {11},
pages = {1328--1331},
abstract = {Trends in oxygen reduction activity of Pd/Cu bimetallic random alloy nanoparticles are determined with calculations of oxygen binding for a range of compositions. A reduction in the average oxygen binding is found as Cu is added to Pd, indicating an increase in catalytic activity up to a peak at 1:1 Pd/Cu ratio. Calculations show that Cu reduces the Pd-O binding energy and Pd increases the Cu-O binding energy. These changes are understood in terms of charge transfer from Pd to Cu, lowering the d-band center of Pd and raising that of Cu. The peak in activity occurs because these two effects not equivalent. A greater overlap between the d-states of Pd and the adsorbed oxygen makes the reduction in binding at Pd more significant than the increase in binding at Cu. We present a simple model of the average binding energy that can generally predict activity trends in random alloys. textcopyright 2011 American Chemical Society.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Yue Pan, Shiyu Zhen, Xiaozhi Liu, Mengshu Ge, Jianxiong Zhao, Lin Gu, Dan Zhou, Liang Zhang, Dong Su, "Looping metal-support interaction in heterogeneous catalysts during redox reactions", Nat Commun, 16, 8627, 2025.
@article{Pan2025b,
title = {Looping metal-support interaction in heterogeneous catalysts during redox reactions},
author = {Yue Pan and Shiyu Zhen and Xiaozhi Liu and Mengshu Ge and Jianxiong Zhao and Lin Gu and Dan Zhou and Liang Zhang and Dong Su},
doi = {10.1038/s41467-025-63646-1},
issn = {2041-1723},
year = {2025},
date = {2025-12-00},
urldate = {2025-12-00},
journal = {Nat Commun},
volume = {16},
number = {8627},
publisher = {Springer Science and Business Media LLC},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Haiyang Lan, Zixuan Xue, Jiakun Sun, Jiaming Chu, Jiangyuan Feng, Weitao Jin, Zixian Wang, Ruiqing Song, Pengfei Yan, Liang Zhang, Yucun Zhou, Xiaofeng Ye, Juan Zhou, "An in-situ generated multiphase catalyst for active and durable ammonia protonic ceramic fuel cells", Chemical Engineering Journal, 523, 168483, 2025.
@article{Lan2025,
title = {An in-situ generated multiphase catalyst for active and durable ammonia protonic ceramic fuel cells},
author = {Haiyang Lan and Zixuan Xue and Jiakun Sun and Jiaming Chu and Jiangyuan Feng and Weitao Jin and Zixian Wang and Ruiqing Song and Pengfei Yan and Liang Zhang and Yucun Zhou and Xiaofeng Ye and Juan Zhou},
doi = {10.1016/j.cej.2025.168483},
issn = {1385-8947},
year = {2025},
date = {2025-11-01},
urldate = {2025-11-01},
journal = {Chemical Engineering Journal},
volume = {523},
number = {168483},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Hongyang Su, Jing Chai, Zixuan Guan, Hendrik Bluhm, Ziyun Zhang, Yidan Cao, Zhi Liu, Yuan-Hua Lin, William C. Chueh, Liang Zhang, Di Chen, "Tuning the f Band for Enhanced Surface Redox in Strained Rare Earth Oxides", J. Am. Chem. Soc., 2025.
@article{Su2025,
title = {Tuning the f Band for Enhanced Surface Redox in Strained Rare Earth Oxides},
author = {Hongyang Su and Jing Chai and Zixuan Guan and Hendrik Bluhm and Ziyun Zhang and Yidan Cao and Zhi Liu and Yuan-Hua Lin and William C. Chueh and Liang Zhang and Di Chen},
doi = {10.1021/jacs.5c08872},
issn = {1520-5126},
year = {2025},
date = {2025-10-24},
urldate = {2025-10-24},
journal = {J. Am. Chem. Soc.},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Shiyu Zhen, Yong Wang, Yuhan Wang, Jianhao Wang, Xiangfu Niu, Kailang Li, Dong Su, Haohong Duan, Baorui Jia, Mingli Qin, Liang Zhang, "Machine-learning guided design of non-precious-metal high-entropy electrocatalysts for alkaline hydrogen evolution", eScience, 2025, 100484.
@article{nokey,
title = {Machine-learning guided design of non-precious-metal high-entropy electrocatalysts for alkaline hydrogen evolution},
author = {Shiyu Zhen, Yong Wang, Yuhan Wang, Jianhao Wang, Xiangfu Niu, Kailang Li, Dong Su, Haohong Duan, Baorui Jia, Mingli Qin and Liang Zhang},
doi = {10.1016/j.esci.2025.100484},
year = {2025},
date = {2025-10-20},
urldate = {2025-12-29},
journal = {eScience},
pages = {100484},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Jing Chai, Lixiang Qian, Yucun Zhou, Jianqiu Li, Liang Zhang, "The Direct Ammonia Oxidation Reaction Mechanism and Selectivity on Ni-YSZ Anodes in Solid Oxide Fuel Cells", ACS Catalysis, 2025, 18167–18176.
@article{nokeyf,
title = {The Direct Ammonia Oxidation Reaction Mechanism and Selectivity on Ni-YSZ Anodes in Solid Oxide Fuel Cells},
author = {Jing Chai, Lixiang Qian, Yucun Zhou, Jianqiu Li, Liang Zhang},
doi = {10.1021/acscatal.5c05016},
year = {2025},
date = {2025-10-18},
urldate = {2025-12-30},
journal = {ACS Catalysis},
pages = {18167–18176},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiangfu Niu, Yanjun Chen, Mingze Sun, Satoshi Nagao, Yuki Aoki, Zhiqiang Niu, Liang Zhang, "Bayesian-Learning-Assisted Catalyst Discovery for Efficient Iridium Utilization in Electrochemical Water Splitting", Science Advances, 11, 34, 2025, eadw0894.
@article{nokey,
title = {Bayesian-Learning-Assisted Catalyst Discovery for Efficient Iridium Utilization in Electrochemical Water Splitting},
author = {Xiangfu Niu, Yanjun Chen, Mingze Sun, Satoshi Nagao, Yuki Aoki, Zhiqiang Niu, Liang Zhang},
url = {https://www.science.org/doi/10.1126/sciadv.adw0894},
year = {2025},
date = {2025-08-20},
urldate = {2025-08-20},
journal = {Science Advances},
volume = {11},
issue = {34},
pages = {eadw0894},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Shuzhen Li, Xuan Luo, Yueshuai Wang, Chaowei Wang, Guizhen Zhang, Huixin Xiang, Yong Yan, Xiaoxing Ke, Yue Lu, Chuanhao Yao, Hongyi Li, Liang Zhang, Ge Chen, "Strong Oxide-Support Interaction Induced Thermal Stabilization of Pt Single Atoms for Durable Catalytic CO Oxidation", Angewandte Chemie International Edition, e202504551, 2025, e202504551.
@article{https://doi.org/10.1002/anie.202504551,
title = {Strong Oxide-Support Interaction Induced Thermal Stabilization of Pt Single Atoms for Durable Catalytic CO Oxidation},
author = {Shuzhen Li and Xuan Luo and Yueshuai Wang and Chaowei Wang and Guizhen Zhang and Huixin Xiang and Yong Yan and Xiaoxing Ke and Yue Lu and Chuanhao Yao and Hongyi Li and Liang Zhang and Ge Chen},
url = {https://onlinelibrary.wiley.com/doi/abs/10.1002/anie.202504551},
doi = {https://doi.org/10.1002/anie.202504551},
year = {2025},
date = {2025-08-11},
urldate = {2025-08-11},
journal = {Angewandte Chemie International Edition},
volume = {n/a},
number = {e202504551},
pages = {e202504551},
abstract = {Abstract Supported metal nanoparticle catalysts often suffer from sintering-induced size-dependent deactivation, limiting their high-temperature applications. Although high-temperature redispersion offers a potential solution, this strategy remains restricted to reducible support materials, severely limiting the selection of catalyst supports with versatile compositions and tunable functionalities. Here, we engineer cationic vacancies at Al2O3-La2O3 interface via strong oxide-support interaction (SOSI)—driven interfacial reconstruction during calcination. The vacancy-mediated confinement effect dynamically intercepts migrating Pt species, enabling the construction of Al2O3-Pt1-La2O3 structure with precisely defined coordination environments. The resulting catalyst achieves complete CO conversion at 145 °C and maintains stability with minimal decline after a 6-h treatment at 1100 °C in air with 10% steam. This interfacial engineering strategy proves universal, as demonstrated by ZrO2-La2O3 counterparts. Our findings break the reducibility dependency in traditional single-atom catalysts (SACs) stabilization by establishing oxide–oxide interface as universal anchoring platforms, which expands the design space of industrial-grade SACs beyond conventional reducible oxides.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Wenxia Chen, Zhiyi Sun, Shiyu Zhen, Yujie Wang, Jingqi Sun, Meng Liu, Wen‐Jun Han, Lanmei Lai, Wei Wei, Liang Zhang, Wenxing Chen, "Tuning Asymmetric S‐Bridged Cu─Co Dual Sites at Atomic‐Level for Efficient Ammonia Electrosynthesis", Adv Funct Materials, 2025.
@article{Chen2025b,
title = {Tuning Asymmetric S‐Bridged Cu─Co Dual Sites at Atomic‐Level for Efficient Ammonia Electrosynthesis},
author = {Wenxia Chen and Zhiyi Sun and Shiyu Zhen and Yujie Wang and Jingqi Sun and Meng Liu and Wen‐Jun Han and Lanmei Lai and Wei Wei and Liang Zhang and Wenxing Chen},
doi = {10.1002/adfm.202509200},
issn = {1616-3028},
year = {2025},
date = {2025-08-05},
urldate = {2025-08-05},
journal = {Adv Funct Materials},
publisher = {Wiley},
abstract = {<jats:title>Abstract</jats:title><jats:p>Electrochemical nitrate reduction reaction (NO<jats:sub>3</jats:sub>RR) for ammonia production holds immense promise as an environmentally benign strategy. Nevertheless, the process faces inherent limitations by sluggish kinetics of the eight‐electron transfer and multiple competing reactions. Here, an asymmetric S‐bridged Cu, Co dual‐atom (Cu─S─Co) catalyst (CuCo‐SNC) is creatively reported by leveraging the abundant disulfide bond capture and chelation capabilities of wool keratin. Benefiting from the charge regulation effect between the metal sites and S‐bridged atoms, the CuCo‐SNC catalyst exhibits an optimal Faradaic efficiency of 97.8% at −0.3 V (vs RHE) and a remarkable NH<jats:sub>3</jats:sub> yield rate of 0.88 mmol h<jats:sup>−1</jats:sup> cm<jats:sup>−2</jats:sup> at −0.6 V (vs RHE). The assembled Zn‐NO<jats:sub>3</jats:sub><jats:sup>−</jats:sup> battery shows a power density of 7.99 mW cm<jats:sup>−2</jats:sup> and exceptional cycling stability. Furthermore, in situ characterization and theoretical analysis reveal that the S‐bridge breaks the electron balance between Cu and Co, effectively modulating the charge state of the Cu─Co site, which boosts electrons transfer from Cu to Co site through the S‐bridge, thereby promoting the efficient conversion of nitrate ions (NO<jats:sub>3</jats:sub><jats:sup>−</jats:sup>) at Co─Cu bimetallic sites. This asymmetric dual atom catalyst provides a novel perspective for the development of advanced electrocatalytic technologies in ammonia synthesis.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Cunyuan Gao, Yutong Wang, Shiyu Zhen, Jinhua Zhan, Liang Zhang, Bin Cai, "Rapid Synthesis of Carbon-Supported Ir–Ni Bimetallic Nanoparticles for Efficient Water Oxidation", J. Phys. Chem. C, 129, 28, 2025, 12796-12803.
@article{Gao2025b,
title = {Rapid Synthesis of Carbon-Supported Ir–Ni Bimetallic Nanoparticles for Efficient Water Oxidation},
author = {Cunyuan Gao and Yutong Wang and Shiyu Zhen and Jinhua Zhan and Liang Zhang and Bin Cai},
doi = {10.1021/acs.jpcc.5c03813},
issn = {1932-7455},
year = {2025},
date = {2025-07-17},
urldate = {2025-07-17},
journal = {J. Phys. Chem. C},
volume = {129},
number = {28},
pages = {12796--12803},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiangfu Niu, Shiyu Zhen, Rui Zhang, Jianqiu Li, Liang Zhang, "Unraveling Disorder-to-Order Transitions and Chemical Ordering in PtCoM Ternary Alloys Using Machine Learning Potential", Chemical Science , 16, 33, 2025, 14884-14893.
@article{nokey,
title = {Unraveling Disorder-to-Order Transitions and Chemical Ordering in PtCoM Ternary Alloys Using Machine Learning Potential},
author = {Xiangfu Niu, Shiyu Zhen, Rui Zhang, Jianqiu Li, Liang Zhang},
url = {https://doi.org/10.1039/D5SC04043D},
year = {2025},
date = {2025-07-15},
urldate = {2025-07-15},
journal = {Chemical Science },
volume = {16},
issue = {33},
pages = {14884-14893},
note = {selected},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Kai Chen, Lixiang Qian, Haohong Duan, Rui Zhang, Jianqiu Li, Liang Zhang, "Impact of surface oxygen functionalization on local oxygen transport resistance at the fuel cell cathode", Chemical Engineering Science, 313, 2025, 121745.
@article{Chen2025,
title = {Impact of surface oxygen functionalization on local oxygen transport resistance at the fuel cell cathode},
author = {Kai Chen and Lixiang Qian and Haohong Duan and Rui Zhang and Jianqiu Li and Liang Zhang},
url = {https://www.sciencedirect.com/science/article/pii/S0009250925005688},
issn = {0009-2509},
year = {2025},
date = {2025-07-01},
urldate = {2025-07-01},
journal = {Chemical Engineering Science},
volume = {313},
pages = {121745},
abstract = {The high oxygen transport resistance at the carbon/ionomer interface is one of the primary limitations for proton exchange membrane fuel cells (PEMFCs) with low platinum (Pt) loadings. This study uses molecular dynamics (MD) simulations to explore how oxygen-containing functional groups on carbon supports influence ionomer distribution and oxygen permeation. Results show that C=O and C-O-C groups prevent the dense overlay of ionomer backbones, enhancing phase segregation and improving both oxygen diffusion and solubility. By optimizing surface oxygen species and ratios (4 %–8%), these groups significantly reduce local oxygen transport resistance (RLocal). This work offers new insights for designing modified carbon supports to improve PEMFC performance by enhancing oxygen permeation and reducing RLocal.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xue Jia, Tianyi Wang, Di Zhang, Xuan Wang, Heng Liu, Liang Zhang, Hao Li, "Advancing electrocatalyst discovery through the lens of data science: State of the art and perspectives", Journal of Catalysis, 447, 2025, 116162.
@article{Jia2025,
title = {Advancing electrocatalyst discovery through the lens of data science: State of the art and perspectives},
author = {Xue Jia and Tianyi Wang and Di Zhang and Xuan Wang and Heng Liu and Liang Zhang and Hao Li},
doi = {10.1016/j.jcat.2025.116162},
issn = {0021-9517},
year = {2025},
date = {2025-07-00},
urldate = {2025-07-00},
journal = {Journal of Catalysis},
volume = {447},
pages = {116162},
publisher = {Elsevier BV},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Hongtao Wang, Kailang Li, Miguel Lopez-Haro, Carlo Marini, Zhe He, Minghao Gao, Liang Zhang, Lichen Liu, "Subnanometer Pt Catalysts Encapsulated in MEL Zeolite Mesocrystals for H2 Production from Methylcyclohexane Dehydrogenation", Journal of the American Chemical Society, 2025.
@article{Wang2025b,
title = {Subnanometer Pt Catalysts Encapsulated in MEL Zeolite Mesocrystals for H2 Production from Methylcyclohexane Dehydrogenation},
author = {Hongtao Wang and Kailang Li and Miguel Lopez-Haro and Carlo Marini and Zhe He and Minghao Gao and Liang Zhang and Lichen Liu},
url = {https://doi.org/10.1021/jacs.5c04618},
doi = {10.1021/jacs.5c04618},
issn = {0002-7863},
year = {2025},
date = {2025-06-11},
urldate = {2025-06-11},
journal = {Journal of the American Chemical Society},
publisher = {American Chemical Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Helai Huang, Mingze Sun, Kai Chen, Yizhen Che, Xin Tang, Zhengwen Li, Kaiqi Nie, Shuairen Qian, Jinjie Fang, Haiyong Wang, Yanfen Wu, Qikun Hu, Yuqi Wang, Xiaohang Sun, Junliang He, Yu-Xiao Zhang, Zhongbin Zhuang, Liang Zhang, Zhiqiang Niu, "Unlocking the Potential of Mn‐based Catalyst for Durable Two‐electron Oxygen Reduction in Acid at High Current Densities", Angew Chem Int Ed, 2025.
@article{Huang2025,
title = {Unlocking the Potential of Mn‐based Catalyst for Durable Two‐electron Oxygen Reduction in Acid at High Current Densities},
author = {Helai Huang and Mingze Sun and Kai Chen and Yizhen Che and Xin Tang and Zhengwen Li and Kaiqi Nie and Shuairen Qian and Jinjie Fang and Haiyong Wang and Yanfen Wu and Qikun Hu and Yuqi Wang and Xiaohang Sun and Junliang He and Yu-Xiao Zhang and Zhongbin Zhuang and Liang Zhang and Zhiqiang Niu},
doi = {10.1002/anie.202511844},
issn = {1521-3773},
year = {2025},
date = {2025-06-10},
urldate = {2025-06-10},
journal = {Angew Chem Int Ed},
publisher = {Wiley},
abstract = {<jats:p>Electrochemical synthesis of H2O2 by two‐electron oxygen reduction (2e− ORR) often shows limited stability at high current densities in acidic media. Mn‐based catalysts have been demonstrated highly stable for four‐electron ORR thanks to their intrinsically low rate constant for Fenton‐like reactions. However, their activity toward acidic 2e− ORR remains low because of too strong adsorption to *OOH. Here, we report a diatomic Mn catalyst with high‐spin MnII centers to enable high onset potential (0.69 V), high selectivity (> 90%) and outstanding stability (240 h under 300 mA cm−2) towards H2O2 electrosynthesis in acid. Theoretical calculations and in situ spectroscopies reveal that the diatomic Mn sites have downshifted d‐band center and thus weakened adsorption strength for *OOH. Moreover, the inertia of the MnII sites toward the troublesome Fenton‐like reactions leads to the long‐term stability at high current densities. We further demonstrate the functionalization of waste polyethylene (PE) using the high‐concentration H2O2 as produced, which provides a sustainable route toward on‐site upcycling of plastic waste.</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Yue Pan, Xiaozhi Liu, Shiyu Zhen, Jianxiong Zhao, Yuhan Wang, Mengshu Ge, Jinrui Zhang, Ying Pan, Lin Gu, Liang Zhang, Dan Zhou, Dong Su, "Alloying Effects on Iron Oxide Redox Pathways: Insights into Sustainable Hydrogen-Based Reduction", J. Phys. Chem. Lett., 16, 22, 2025, 5506-5514.
@article{Pan2025,
title = {Alloying Effects on Iron Oxide Redox Pathways: Insights into Sustainable Hydrogen-Based Reduction},
author = {Yue Pan and Xiaozhi Liu and Shiyu Zhen and Jianxiong Zhao and Yuhan Wang and Mengshu Ge and Jinrui Zhang and Ying Pan and Lin Gu and Liang Zhang and Dan Zhou and Dong Su},
doi = {10.1021/acs.jpclett.5c00954},
issn = {1948-7185},
year = {2025},
date = {2025-06-05},
urldate = {2025-06-05},
journal = {J. Phys. Chem. Lett.},
volume = {16},
number = {22},
pages = {5506--5514},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Yan Zhang, Fuhua Li, Shuwei Li, Zedong Zhang, Zhiyi Sun, Yuhai Dou, Rui Su, Yahe Wu, Liang Zhang, Wenxing Chen, Dingsheng Wang, Yadong Li, "Asymmetric Dual-Atomic Catalyst with Axial Chloride Coordination for Efficient Oxygen Reduction Reaction", Advanced Materials, 2025, 2507478.
@article{https://doi.org/10.1002/adma.202507478,
title = {Asymmetric Dual-Atomic Catalyst with Axial Chloride Coordination for Efficient Oxygen Reduction Reaction},
author = {Yan Zhang and Fuhua Li and Shuwei Li and Zedong Zhang and Zhiyi Sun and Yuhai Dou and Rui Su and Yahe Wu and Liang Zhang and Wenxing Chen and Dingsheng Wang and Yadong Li},
url = {https://advanced.onlinelibrary.wiley.com/doi/abs/10.1002/adma.202507478},
doi = {https://doi.org/10.1002/adma.202507478},
year = {2025},
date = {2025-05-29},
urldate = {2025-05-29},
journal = {Advanced Materials},
volume = {n/a},
number = {n/a},
pages = {2507478},
abstract = {Abstract Low-platinum-group metal (low-PGM) catalysts play a crucial role in reducing the cost of proton exchange membrane fuel cells (PEMFCs). Dual-atomic catalysts offer valuable solutions due to their exceptional performance. This work explores the application of axial Cl-coordinated Pt─Co dual atoms on N-doped graphitic carbon (Pt1Co1/NC─Cl) catalysts utilizing PtCo dual-atomic catalysts, demonstrating their ability to significantly enhance the acidic oxygen reduction reaction (ORR) catalytic performance of conventional PtCo catalysts. The half-wave potential (E1/2) reaches 0.841 V in a 0.1 M HClO4 solution, and only a reduction of 12 mV in E1/2 is observed after 5000 cycles. Axial Cl proves to be resistant to removal during the electroreduction reaction. Consequently, the use of heteroatom-modulated asymmetric structures can greatly improve the performance of Pt-based catalysts. Incorporating nonmetallic synergistic Pt-group metals presents a promising solution for achieving high-performance Low-PGMs.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

An-Zhen Li, Xiongbo Wang, Shuwei Li, Bo-Jun Yuan, Xi Wang, Ruo-Pu Li, Liang Zhang, Bi-Jie Li, Haohong Duan, "Direct Electrooxidation of Ethylene to Ethylene Glycol over 90% Faradaic Efficiency Enabled by Cl– Modification of the Pd Surface", J. Am. Chem. Soc., 147, 12, 2025, 10493-10503.
@article{Li2025,
title = {Direct Electrooxidation of Ethylene to Ethylene Glycol over 90% Faradaic Efficiency Enabled by Cl^{–} Modification of the Pd Surface},
author = {An-Zhen Li and Xiongbo Wang and Shuwei Li and Bo-Jun Yuan and Xi Wang and Ruo-Pu Li and Liang Zhang and Bi-Jie Li and Haohong Duan},
doi = {10.1021/jacs.4c18345},
issn = {1520-5126},
year = {2025},
date = {2025-03-26},
urldate = {2025-03-26},
journal = {J. Am. Chem. Soc.},
volume = {147},
number = {12},
pages = {10493--10503},
publisher = {American Chemical Society (ACS)},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Xiangfu Niu, Shuwei Li, Zheyu Zhang, Haohong Duan, Rui Zhang, Jianqiu Li, Liang Zhang, "Accelerated Optimization of Compositions and Chemical Ordering for Bimetallic Alloy Catalysts Using Bayesian Learning", ACS Catalysis, 2025, 4374-4383.
@article{Niu2025,
title = {Accelerated Optimization of Compositions and Chemical Ordering for Bimetallic Alloy Catalysts Using Bayesian Learning},
author = {Xiangfu Niu and Shuwei Li and Zheyu Zhang and Haohong Duan and Rui Zhang and Jianqiu Li and Liang Zhang},
url = {https://doi.org/10.1021/acscatal.5c00467},
doi = {10.1021/acscatal.5c00467},
year = {2025},
date = {2025-02-26},
urldate = {2025-02-26},
journal = {ACS Catalysis},
pages = {4374-4383},
publisher = {American Chemical Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}

Ze-Cheng Yao, Jing Chai, Tang Tang, Liang Ding, Zhe Jiang, Jiaju Fu, Xiaoxia Chang, Bingjun Xu, Liang Zhang, Jin-Song Hu, Li-Jun Wan, "Manipulating hydrogenation pathways enables economically viable electrocatalytic aldehyde-to-alcohol valorization", Proc. Natl. Acad. Sci. U.S.A., 122, 8, 2025.
@article{Yao2025,
title = {Manipulating hydrogenation pathways enables economically viable electrocatalytic aldehyde-to-alcohol valorization},
author = {Ze-Cheng Yao and Jing Chai and Tang Tang and Liang Ding and Zhe Jiang and Jiaju Fu and Xiaoxia Chang and Bingjun Xu and Liang Zhang and Jin-Song Hu and Li-Jun Wan},
doi = {10.1073/pnas.2423542122},
issn = {1091-6490},
year = {2025},
date = {2025-02-25},
urldate = {2025-02-25},
journal = {Proc. Natl. Acad. Sci. U.S.A.},
volume = {122},
number = {8},
publisher = {Proceedings of the National Academy of Sciences},
abstract = {<jats:p>
Electrocatalytic reduction (ECR) of furfural represents a sustainable route for biomass valorization. Unfortunately, traditional Cu-catalyzed ECR suffers from diversified product distribution and industrial-incompatible production rates, mainly caused by the intricate mechanism−performance relationship. Here, we manipulate hydrogenation pathways on Cu by introducing ceria as an auxiliary component, which enables the mechanism switching from proton-coupled electron transfer to electrochemical hydrogen-atom transfer (HAT) and thus high-speed furfural-to-furfuryl alcohol electroconversion. Theoretical and kinetic analyses show that oxygen-vacancy-rich ceria delivers an efficient formation−diffusion−hydrogenation chain of H* by diminishing H* adsorption. Spectroscopic characterizations indicate that Cu/ceria interfacial perimeter enriches the local furfural, synergistically lowering the barrier of the rate-determining HAT step across the perimeter. Our Cu/ceria catalyst realizes high-rate HAT-dominated ECR for electrosynthesis of single-product furfuryl alcohol, achieving a high production rate of 19.1 ± 0.4 mol h
<jats:sup>−1</jats:sup>
m
<jats:sup>−2</jats:sup>
and a Faradaic efficiency of 97 ± 1% at an economically viable partial current density of over 0.1 A cm
<jats:sup>−2</jats:sup>
. Our results demonstrate a highly efficient route for biofeedstock valorization with enhanced techno-economic feasibility.
</jats:p>},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Electrocatalytic reduction (ECR) of furfural represents a sustainable route for biomass valorization. Unfortunately, traditional Cu-catalyzed ECR suffers from diversified product distribution and industrial-incompatible production rates, mainly caused by the intricate mechanism−performance relationship. Here, we manipulate hydrogenation pathways on Cu by introducing ceria as an auxiliary component, which enables the mechanism switching from proton-coupled electron transfer to electrochemical hydrogen-atom transfer (HAT) and thus high-speed furfural-to-furfuryl alcohol electroconversion. Theoretical and kinetic analyses show that oxygen-vacancy-rich ceria delivers an efficient formation−diffusion−hydrogenation chain of H* by diminishing H* adsorption. Spectroscopic characterizations indicate that Cu/ceria interfacial perimeter enriches the local furfural, synergistically lowering the barrier of the rate-determining HAT step across the perimeter. Our Cu/ceria catalyst realizes high-rate HAT-dominated ECR for electrosynthesis of single-product furfuryl alcohol, achieving a high production rate of 19.1 ± 0.4 mol h
<jats:sup>−1</jats:sup>
m
<jats:sup>−2</jats:sup>
and a Faradaic efficiency of 97 ± 1% at an economically viable partial current density of over 0.1 A cm
<jats:sup>−2</jats:sup>
. Our results demonstrate a highly efficient route for biofeedstock valorization with enhanced techno-economic feasibility.
</jats:p>

Xiaochen Wang, Ning Zhang, Huishan Shang, Haojie Duan, Zhiyi Sun, Lili Zhang, Yuanting Lei, Xuan Luo, Liang Zhang, Bing Zhang, Wenxing Chen, "Precisely designing asymmetrical selenium-based dual-atom sites for efficient oxygen reduction", Nature Communications, 16, 1, 2025, 470.
@article{Wang2025,
title = {Precisely designing asymmetrical selenium-based dual-atom sites for efficient oxygen reduction},
author = {Xiaochen Wang and Ning Zhang and Huishan Shang and Haojie Duan and Zhiyi Sun and Lili Zhang and Yuanting Lei and Xuan Luo and Liang Zhang and Bing Zhang and Wenxing Chen},
url = {https://doi.org/10.1038/s41467-025-55862-6},
doi = {10.1038/s41467-025-55862-6},
issn = {2041-1723},
year = {2025},
date = {2025-01-07},
urldate = {2025-01-07},
journal = {Nature Communications},
volume = {16},
number = {1},
pages = {470},
abstract = {Owing to their synergistic interactions, dual-atom catalysts (DACs) with well-defined active sites are attracting increasing attention. However, more experimental research and theoretical investigations are needed to further construct explicit dual-atom sites and understand the synergy that facilitates multistep catalytic reactions. Herein, we precisely design a series of asymmetric selenium-based dual-atom catalysts that comprise heteronuclear SeN2–MN2 (Mþinspace=þinspaceFe, Mn, Co, Ni, Cu, Mo, etc.) active sites for the efficient oxygen reduction reaction (ORR). Spectroscopic characterisation and theoretical calculations revealed that heteronuclear selenium atoms can efficiently polarise the charge distribution of other metal atoms through short-range regulation. In addition, compared with the Se or Fe single-atom sites, the SeFe dual-atom sites facilitate a reduction in the conversion energy barrier from *O to *OH via the coadsorption of *O intermediates. Among these designed selenium-based dual-atom catalysts, selenium-iron dual-atom catalysts achieves superior alkaline ORR performance, with a half-wave potential of 0.926þinspaceV vs. a reversible hydrogen electrode. In addition, the SeN2–FeN2-based Zn–air battery has a high specific capacity (764.8þinspacemAhþinspaceg−1) and a maximum power density (287.2þinspacemWþinspacecm−2). This work may provide a good perspective for designing heteronuclear DACs to improve ORR efficiency.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}